Metabolism is the sum total of the various chemical processes by which food is utilised by a living organism to provide energy, growth substances and cell repair.
Metabolism has two parts:
(i) Degradation of absorbed food substances into smaller molecules, i.e. catabolism, and
(ii) The biosynthesis of complex molecules from simpler components, i.e., anabolism. Catabolic reactions are exothermic, and anabolic reactions are endothermic. Any chemical compound that is involved in a metabolic reaction is referred to as a metabolite.
ADVERTISEMENTS:
The whole animal world derives its foodstuffs from plants. Plants obtain water, inorganic salts and nitrogenous compounds from the soil, land CO2 and O2. With these raw materials plants synthesise carbohydrate, lipids and proteins.
Sunlight supplies necessary energy for different reactions in the plant body. Animals, therefore, obtain carbohydrates, lipids and proteins from plants as their metabolite.
Carbohydrate Metabolism:
Starch is the principal carbohydrate ingested by animals. It is then digested in the alimentary canal. The ultimate products of starch digestion are mainly three monosaccharide units—glucose, fructose and galactose.
ADVERTISEMENTS:
These monosaccharides are then absorbed through the wall of the small intestine into the blood stream, i.e., portal vein. Portal vein carries these monosaccharides to liver, where galactose and fructose are enzymatically converted to glucose.
Glucose is the only sugar that circulates in the blood, hence the name blood sugar. Therefore, from liver glucose comes to the general circulation and reaches various tissues for various purposes. In the tissues, it may be oxidised to form CO2 and H2O, or may be converted to fat or muscle glycogen. All these events occur through different metabolic pathways. These pathways are described here.
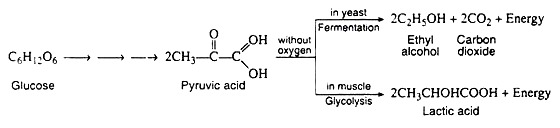
Embeden-Meyerhof Pathway (EMP)/Anaerobic Glycolysis:
A metabolic pathway is represented by a type of flow diagram that enables us to explain how an organism converts a certain reactant into a desired end product. In the 1930s, the German biochemists G. Embden and O. Meyerhof elucidated the sequence of reactions by which glycogen and glucose are degraded in the absence of oxygen (anaerobic conditions) to pyruvic acid.
ADVERTISEMENTS:
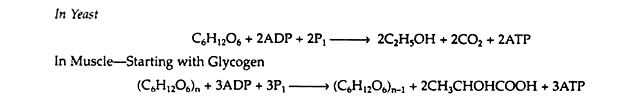
It was discovered that the two apparently dissimilar processes of alcoholic fermentation in yeast and muscle contraction in animals proceed by the same pathway as far as pyruvic acid. In the absence of oxygen, muscle cells (and certain other animal tissues) covert pyruvic acid into lactic acid, but under similar conditions, the enzymes in yeast covert the pyruvic acid to ethyl alcohol and carbon dioxide.
The former process is known as glycolysis, and the latter as fermentation.
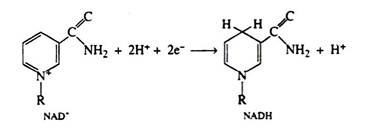
It is important to notice that the conversion of glucose to pyruvic acid represents an oxidation reaction (that is, C6H12O6 → 2CH3COCOOH + 4H+ + 4e−), and yet oxygen is not required for the process to occur. We shall see that the outstanding characteristic of the Embden-Meyerhof pathway is the utilization of the coenzyme NAD+ as the electron acceptor (oxidizing agent). Thus, glycolysis and fermentation differ only in the eventual fate of the pyruvic acid, and hence in the means employed for the regeneration of the NAD+.
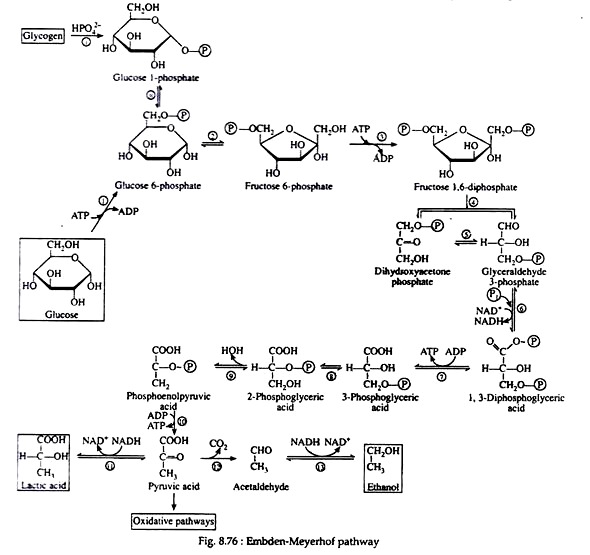
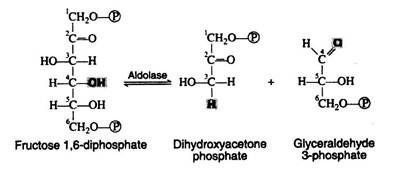
A summary of the reactions and the metabolites involved in the Embden Meyer- half pathway is given in Fig. 8.76. This sequence of reactions is probably the best understood of all the metabolic pathways. Over a dozen different, specific enzyme molecules act in such a manner that the product of the first enzyme-catalyzed reaction becomes the substrate of the next.
Each of the reactions given in Fig. 8.76 has been assigned a number that corresponds to the discussion that follows. In the discussion, the full name of the pertinent enzymes is given in the body of the text. Only the general names are written above or below the arrows of the biochemical equation.
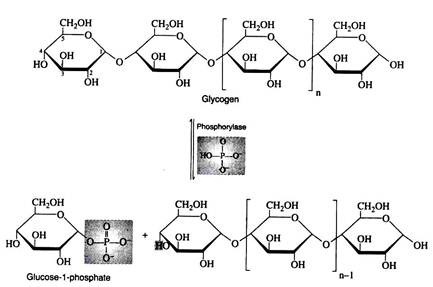
Step i:
Liver and muscle cells contain enzymes called phosphorylates that catalyze the phosphorylitic cleavage of the α-1, 4- glucosidic bonds at the non-reducing end of the glycogen chain to produce glucose 1- phosphate. Although this reaction is reversible, a different enzyme and a different reaction sequence are employed for the biosynthesis of glycogen.
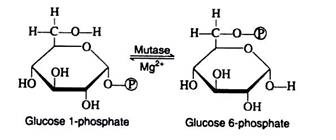
Step ii:
Glucose 1-phosphate and glucose 6-phosphate are readily inter-convertible in the presence of Mg2+ ions and the enzyme phosphoglacomutase. For simplicity, the iso-merisation reaction may be thought of as an intra-molecular transfer of the phosphate group from carbon-1 to carbon-6 and, although this process is reversible, glucose 6- phosphate formation is favoured.
Step 1:
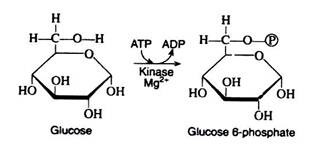
The initial step in the utilisation of glucose by yeast cells and by most animal cells is its phosphorylation to glucose 6-phosphate. The phosphate donor in this reaction is ATP, and the enzyme, which requires Mg2+ ions for its activity, is hexokinase. Hexokinase is not specific for glucose. It can also catalyse the transfer of phosphate for ATP to fructose, mannose, and 2-deoxyglucose.
The reaction is accompanied by an expenditure of energy, since a molecule of ATP is being utilised rather than synthesize. However, this step is necessary for the activation of the glucose molecule. (We shall see that a second molecule of ATP is expended in step 3.
These two initiating molecules of ATP are recovered at a later stage of the pathway.) Reaction 1 is essentially irreversible in the cell; ATP is not formed to an appreciable extent by the reaction of ADP with a simple phosphate ester.
Step 2:
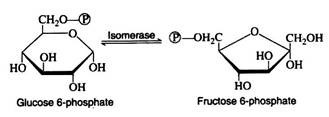
Glucose 6-phosphate is iso-merized to fructose 6-phosphate by the action of the enzyme phosphoglucoisomerase. This enzyme is highly specific for glucose 6-phosphate (e.g., another isomerase is required for the conversion of mannose 6-phosphate to fructose 6-phosphate).
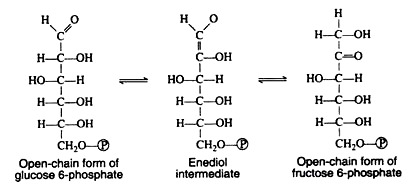
A mechanism for this aldose-ketose transformation involves the formation of an enediol intermediate, and is best understood if the open-chain structures of the sugars are considered:
Step 3:
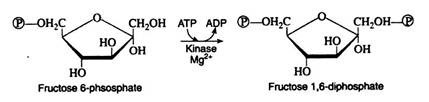
Next follows another phosphorylation reaction, again involving the utilisation of ATP as the phosphate-group donor. The enzyme phosphofructokinase is specific for fructose 6-phosphate and, like hexokinase, requires Mg2+ ions for activity. The reaction is irreversible and represents the nonproductive utilisation of a second molecule of ATP:
Step 4:
Fructose 1, 6-diphosphate is then enzymically cleaved into two molecules of triose phosphate. This cleavage may be regarded as the reverse of an aldol condensation reaction and hence the enzyme has been named aldolase. Again, a better understanding of the reaction is achieved if the fructose 1, 6-diphosphate is written in its open-chain form:
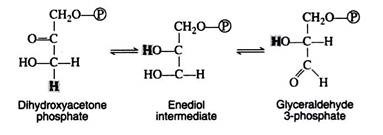
Step 5:
The next step is concerned with the inter-conversion of the triose phosphate. This is essential since only glyceraldehyde 3-phosphate can be further metabolised by the body. If cells were unable to convert dihydroxyacetone phosphate to glyceraldehyde 3-phosphate, then half of the energy stored in the original glucose molecule would be lost and would accumulate in the cell as the non-metabolisable ketotriose phosphate. The enzyme for the iso-merisation reaction is triose phosphate isomerase, and the mechanism involves another enediol intermediate.
A summation of steps 4 and 5 indicates that the enzymes aldolase and triose phosphate isomerase have effectively accomplished the conversion of one molecule of fructose 1, 6-diphosphate into two molecules of glyceraldehyde 3-phosphate. Thus far, the glycolytic pathway has required an energy input in the form of two molecules of ATP but has not yet released any of the energy stored in the glucose.
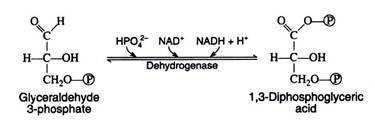
Step 6:
We now come to the first oxidation-reduction reaction in the glycolytic sequence. The conjugated enzyme glyceraldehyde 3-phosphate dehydrogenase contains the coenzyme NAD+ as the prosthetic group. In the process of oxidising the aldehyde to a carboxylic acid, the coenzyme is reduced to NADH.
Notice that the same enzyme also catalyzes a phosphorylation reaction; inorganic phosphate is the phosphate donor. The energy obtained from the oxidation reaction is utilized for the phosphorylation reaction:
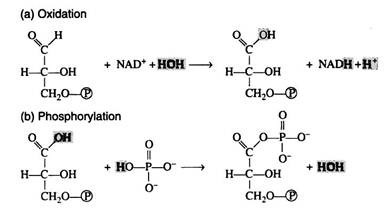
The reaction is more easily understood if it is considered to occur in two steps: (a) oxidation and (b) phosphorylation. Note, however, that this presentation is an oversimplification that in no way implies the actual enzymecatalysed sequence of events:
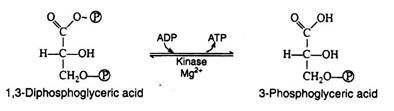
Step 7:
1, 3-Diphosphoglyeerie acid is a high energy compound; it can transfer the phosphate group on carbon-1 directly to a molecule of ADP thus forming a molecule of ATP. The enzyme that catalyses the reaction is phosphoglycerokinase, and like all kinases, it requires Mg2+ ions for activity.
It is in this reaction that ATP is first produced in the pathway. Since ATP is formed by a direct transfer of a phosphate group from a metabolite to ADP, the process is referred to as substrate level phosphorylation, to distinguish it from another ATP synthesizing process to be considered shortly:
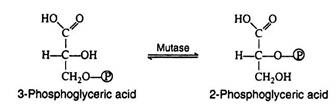
Step 8:
This step in the pathway is very similar to the intra-molecular transfer of phosphate as seen in step ii. The enzyme phosphoglyceromutase catalyses the exchange of a phosphate group from the hydroxyl group of carbon-3 to the hydroxyl group of carbon-2:
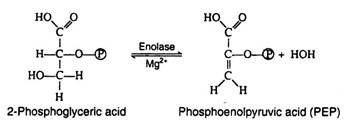
Step 9:
The enzyme enolase, which also requires Mg2+ ions for activity catalyses an alcohol dehydration to produce phosphoenolpyruvic acid, a compound with a high-energy enolic phosphate group:
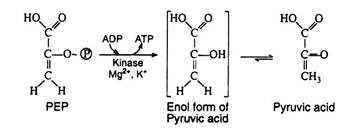
Step 10:
This irreversible step provides a second example of substrate level phosphorylation. The phosphate group of phosphoenolpyruvic acid is transferred to ADP; one molecule of ATP is produced per molecule of PEP. It is likely that the reaction proceeds via the enol form of pyruvic acid, which is unstable with respect to rearrangement to pyruvic acid. The enzyme pyruvate kinase requires both Mg2+ and K+ ions for activity:
Step 11:
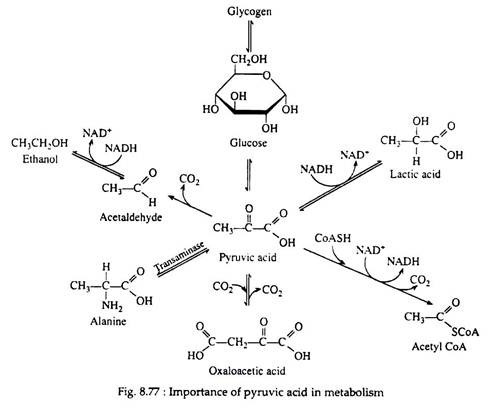
Step I through 10 are identical for glycolysis and fermentation. Pyruvic acid, as we shall see, is the crossroads compound, its metabolic fate is dependent upon the condition (anaerobic or aerobic) and upon the organism under consideration (Fig. 8.77).
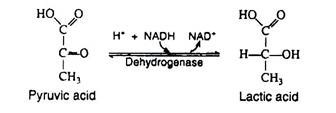
In the presence of NADH, the enzyme lactic acid dehydrogenase catalyses the reduction of pyruvic acid to lactic acid (ketone to secondary alcohol). This reaction, which takes place in muscle cells and in certain other tissues is essential because it regenerates the NAD+ that is needed in step 6. If NAD+ were not replensihed, the cell’s supply of this coenzyme would be swiftly depleted, anaerobic glycolysis would cease, and glyceraldehyde 3-phosphate would accumulate in the cell.
Lactic acid, then, is the end-product of glycolysis, and if there were not some mechanism for its removal, it would accumulate in the muscle cells, raising the level of activity in these cells. Increased acidity impedes muscle performance by causing muscle fatigue and exhaustion. Two processes act to maintain a proper level of lactic acid, both of which require oxygen. The heavy breathing that occurs after strenuous exercise helps to supply this oxygen.
1. 70-80% of the lactic acid diffuses into the bloodstream and is carried to the liver. There it may be oxidised to carbon dioxide and water (via the Krebs cycle) or converted back into glycogen.
2. The remaining 20-20% can be re-oxidized to pyruvic acid, which then enters the Krebs cycle and is further oxidised to carbon dioxide and water during those periods when the muscle cells are afforded an ample supply of oxygen.
Step 12:
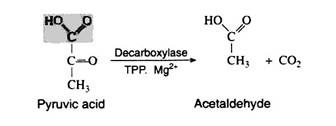
Yeast and other microorganisms that can live in a limited supply of oxygen must also have some means of regenerating NAD+. They do so by first decarboxylating pyruvic acid to acetaldehyde. This reaction, which is catalysed by pyruvic acid decarboxylase, is responsible for CO2 production in yeast (the reason yeast is used in certain baking processes). The enzyme requires the coenzyme thiamine pyrophosphate (TPP) and Mg2+ ions. The decarboxylation reation is irreversible:
Step 13:
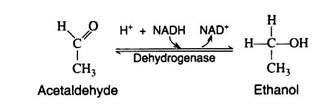
The final step in the fermentation process is catalysed by the enzyme alcohol dehydrogenase. Acetaldehyde is reduced by NADH to ethyl alcohol, thus regenerating NAD+:
Reversal of Glycolysis and Fermentation:
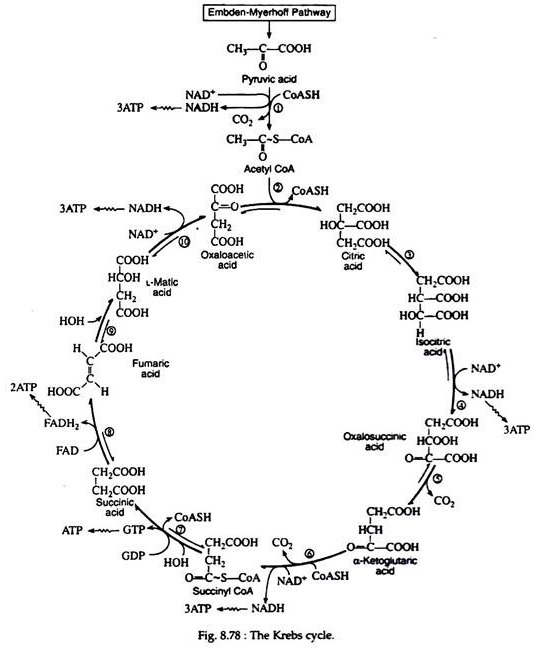
It has been mentioned that several of the degradative steps of the Embden-Meyerhof pathway are not reversible in vivo. (Single arrows are used in Fig. 8.78 to indicate that reactions, i, 1,3,10 and 12 are nonreversible). This seems to be inconsistent with the fact that lactic acid and any of the other intermediates of the glycolytic sequence can be converted back to glycogen provided an energy source is available.
Therefore, other reactions catalysed by different enzymes must necessarily affect a reversal of steps i, 1, 3, and 10. The irreversibility of parts of a metabolic sequence is a common phenomenon; it will be encountered again in aerobic metabolism as well as in the metabolism of lipids and proteins. Catabolic and anabolic processes may have many of the same intermediates and many of the same enzymes, yet their pathways are not identical.
Similarly, more than one pathway may exist for the same process. The separation of degradative and synthetic pathways allows an organism to control one series of reactions without affecting the other. Since we are concerned primarily with reactions that yield energy and produce ATP, we shall not take up the anabolism of carbohydrates.
Energetic of Glycolysis and Fermentation:
The net energy yield in the form of high energy phosphate bonds (moles of ATP) obtained from the anaerobic metabolism of each mole of glucose can be easily calculated from Fig. 8.78.
One mole of ATP is expended in the initial phosphorylation of glucose (step 1):
And a second mole is consumed in the phosphorylation of fructose 6-phosphate (step 3):
Steps 4 and 5 are very significant for these reactions that each mole of six-carbon sugar is transfermed into two moles of the triose phosphate, glyceraldehyde 3-phosphate. In step 7 each mole of triose is converted into one mole of 3-phosphoglyceric acid; one mole of ATP is produced per mole of triose, or two moles of ATP per mole of glucose:
In reaction 10, one mole of ATP is generated for each mole of pyruvic acid that is formed from phosphoenolpyruvic acid; again two moles of ATP are produced for each mole of glucose that entered the pathway:
A summation of all these steps reveals that for every mole of glucose degraded, two moles of ATP are initially consumed and four moles of ATP are ultimately produced. The net production of ATP is thus two moles per mole of glucose converted to lactic acid or to ethanol.
If, however, glycogen is used as the source of glucose, steps i and ii are operative instead of step 1. It would then be necessary to expend only one mole of ATP (in step 3) in order to produce fructose 1, 6-diphosphate, and the net yield of ATP would be three moles per mole of glucose 1-phosphate.
It is known that 7,300 calories of free energy is conserved per molecule of ATP produced. It is also established that the total amount of energy that can theoretically be obtained from the complete oxidation of one mole of glucose is 686,000 calories.
The energy conserved by anaerobic metabolism, then, is only a minute amount of the total energy that is available, i.e., either 2.1% (14,600/686,000) or 3.2% (21,900/686,000). Thus, anaerobic cells extract only a small fraction of the total energy of the glucose molecule, yet this amount is sufficient for their survival. Since they are not nearly as efficient as aerobic cells, it is necessary that they utilise more glucose per unit of time to accomplish the same amount of cellular work.
Aerobic Metabolism:
Krebs Cycle/Tricarboxylic Acid Cycle (TCA cycle)/Citric Acid Cycle:
We have mentioned that the anerobic phase of glucose catabolism ends with the production of lactic acid or ethanol and that it accounts for only a small fraction of the total available energy of the glucose molecule. Aerobic organisms utilise the identical series of glycolytic reactions up to the production of pyruvic acid. Pyruvic acid, however, is not reduced to lactic acid but is oxidised through a number of discrete enzymatic reactions to carbon dioxide and water.
2CH3COCOOH+5O2 → 6CO2+4H2O+~639,000 calories
From the standpoint of energy production, the oxidation of pyruvic acid is of considerable significance because it liberates most of the energy (93%) stored in the glucose molecule. Much of this energy is stored in a chemical form in the high energy phosphate bonds of ATP.
A scheme for the complex series of reaction that effects the oxidation of glucose to carbon dioxide and water was first proposed by Sir Hans Krebs in 1937 (Nobel Prize for Medicine, 1953). Pyruvic acid is first decarboxylated to a two-carbon compound, which then enters a cyclic sequences of reactions known collectively as the Krebs cycle, the tricarboxylic acid cycle, or the citric acid cycle. The Krebs cycle functions to produce ATP and to provide metabolic intermediates for the biosynthesis of needed compounds.
As we shall see in later chapters, the Krebs cycle is not restricted to the metabolism of only carbohydrates. It also plays a vital role in the metabolism of lipids and proteins, and, thus, occurs in almost all cells of higher animals. Every enzyme that participates in the cycle has been identified, and the operation of this metabolic pathway in vivo has been completely verified by the use of isotopic tracers.
Fig. 8.78 is a schematic outline of the Krebs cycle. Each reaction is numbered and the individual steps of the sequence will now be considered in detail.
Step 1:
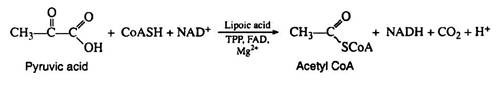
The pyruvic acid formed in the Embden—Meyerhof pathway is not an intermediate in the Krebs cycle. It must first be enzymically decarboxylated and oxidised (oxidative decarboxylation) to yield the extremely important intermediate acetyl coenzyme A. The formation of acetyl CoA from pyruvic acid requires the participation of six cofactors: coenzyme A, thiamine pyrophosphate (TPP), lipoic acid, NAD+, FAD, and Mg2+:
The name given to this multi-enzyme system is pyruvic acid dehydrogenase complex. The mechanism of the reaction is believed to involve four distinct steps, decarboxylation, reductive acetylation, acetyl transfer, and electron transport. The initial decarboxylation step is irreversible; hence the overall conversion of pyruvic acid to acetyl CoA is irreversible.
Step 2:
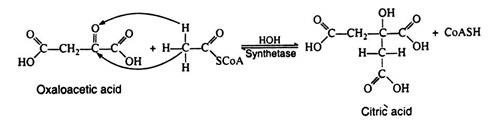
Acetyl CoA is likewise not a true intermediate in the Krebs cycle. It enters the cycle by condensing with the four-carbon dicarboxylic acid oxaloacetic acid, yielding citric acid, the six carbons tricarboxylic acid for which the cycle was named.
Notice that the reaction is very similar to an aldol condensation reaction and for this reason the enzyme was originally referred to as the condensing enzyme. This enzyme is now called citric acid synthetase. The two carbon atoms that originate from acetyl CoA are shown in boldface type here and in subsequent reaction. Note that this step regenerates coenzyme A:
Step 3:
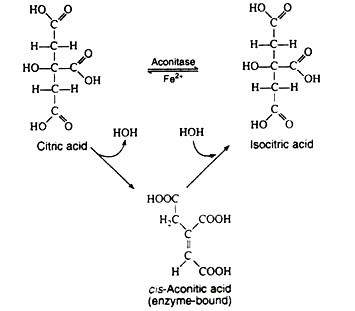
The next step is sometimes considered to be two separate reactions. The single enzyme aconitase catalyzes the successive elimination and reincorporation of a molecule of water. The net result is the isomerisation of citric acid to its less symmetrical isomer, isocitric acid. The intermediate is cis-aconitic acid, and it normally remains bound to the enzyme:
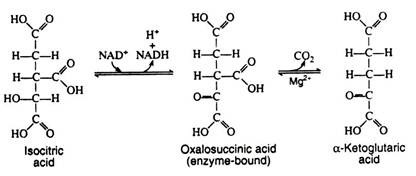
Steps 4 and 5:
The next two steps are usually discussed together because experimental evidence indicates that the intermediate, oxalosuccinic acid, does not exist free but is firmly bound to the surface of the enzyme. Two enzymes, collectively called isocitric dehydrogenase, catalyze the oxidative decarboxylation of isocitric acid to α-ketoglutaric acid.
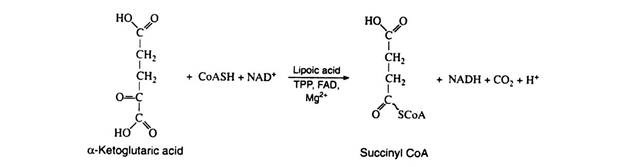
Step 6:
This step is practically identical to step 1; it is catalyzed by another multi-enzyme system known as α-ketoglutaric acid dehydrogenase complex, which requires the same six cofactors as pyruvic acid dehydrogenase. This is the only nonreversible reaction (because of the decarboxylation step) in the Krebs cycle and, as such, prevents the cycle from operating in the reverse direction:
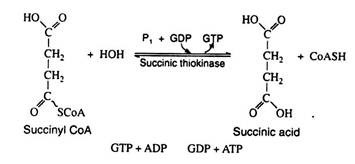
Step 7:
In the reaction the chemical energy of the high-energy thioester bond is conserved by coupling the hydrolysis of succinyl CoA with the formation of guanosine triphosphate (GTP) from guanosine diphosphate (GDP) and inorganic phosphate. This reaction is significant because GTP can react with ADP to generate ATP. Here we have another example of substrate level phosphorylation:
Step 8:
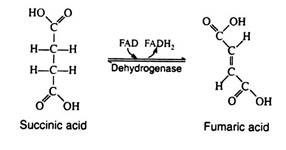
The enzyme succinic acid dehydrogenase catalyses the removal of two hydrogen atoms from succinic acid, thus forming fumaric acid. This dehydrogenation reaction is the only one in the cycle that is catalyzed by the coenzyme FAD rather than by NAD+:
Step 9:
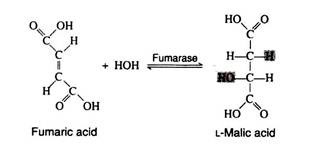
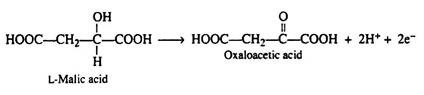
The addition of a molecule of water across the double bond of fumaric acid to form L-malic acid is catalysed by the enzyme fumarase. This enzyme is highly stereospecific; only the L-isomer of malic acid is produced.
Step 10:
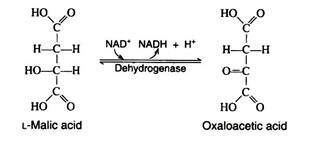
One revolution of the cycle is completed when the dehydrogenation of L- malic acid is brought about by the enzyme malic acid dehydrogenase. This is the fourth oxidation-reduction reaction that utilizes NAD+ as the oxidizing agent:
Respiratory Chain (Electron Transport Chain/System):
We have stated that aerobic metabolism occurs only in the presence of molecular oxygen. It has also been mentioned that the major portion of solar energy stored in carbohydrates is conserved in the process. Yet nowhere in the Krebs cycle has the utilisation of oxygen or the conservation of energy been indicated.
None of the intermediates of the cycle has been shown to be linked to phosphate groups, and no direct synthesis of ATP took place from ADP and inorganic phosphate. The Krebs cycle deals primarily with the fate of the carbon skeleton of pyruvic acid, describing the metabolites involved in its conversion to carbon dioxide and water.
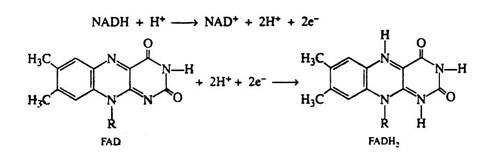
The coenzymes NAD+ and FAD are reduced to NADH and FADH2; no mechanism has yet been indicated for their regeneration. The reduced coenzymes must be re-oxidised if the aerobic phase of carbohydrate metabolism is to continue.
All the enzymes and co-factors that are necessary for the Krebs cycle and for the conservation of energy are localised in the mitochondria. The enzymes contained within the mitochondria are fixed in geometrically specific arrays such that they are capable of functioning as extremely efficient assembly lines.
The sequence of reactions whereby the reduced forms of the coenzymes are re-oxidised (ultimately by molecular oxygen) is known as the respiratory chain or the electron transport chain (ETC).
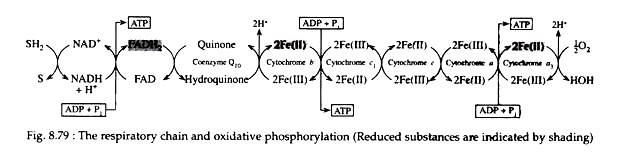
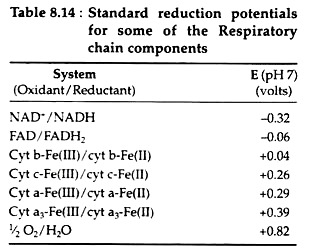
Fig. 8.79 illustrates the respiratory chain in the mitochondria. The sequence in which the electron carriers of the chain operate is determined by their respective reduction potentials. The reduction potentials of the intermediates of the respiratory chain are listed in Table 8.14:
For each molecule of pyruvic acid that is converted to carbon dioxide and water via the Krebs cycle, five dehydrogenation reactions occur. NAD+ serves as the electron acceptor in four of these (steps 1, 4, 6 and 10) and FAD is the oxidising agent in the fifth (step 8).
In Fig. 8.79 SH2 symbolizes the reduced metabolites (i.e., pyruvic acid, iso-citric acid, α-ketoglutaric acid, and malic acid) and S signifies the oxidised metabolites (i.e., acetyl CoA, oxalosuccinic acid, succinyl CoA, and oxaloacetic acid).
We will now examine one of these conversions by writing a balanced half-reaction:
It has been stated that the function of the coenzyme NAD+ is to accept this pair of electrons. We can also write a balanced half-reaction for the reduction of the coenzyme; R—represents the remaining portion of the NAD+ molecule:
Two hydrogen ions and two electrons are removed from the substrate. The NAD+ molecule accepts both electrons and one hydrogen ion; the other hydrogen ion is released into the medium. In the next step of the respiratory chain, both of these hydrogen ions are passed on to the coenzyme FAD, causing its reduction to FADH2. Simultaneously, the reduced form of the coenzyme NADH is re-oxidised, and it is this step that accounts for the regeneration of NAD+. Again we shall depict only the relevant portion of the FAD molecule. Note that in the oxidation of succinic acid to fumaric acid, FAD accepts two hydrogen ions and two electrons directly from the succinic acid:
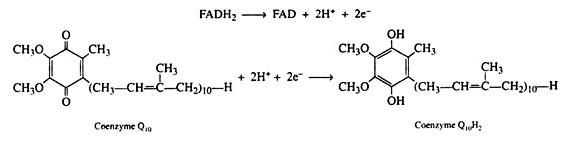
The electrons and the hydrogen ions are then transferred from FADH2 to coenzyme Q(CoQ). This coenzyme is a quinone derivative with a long isoprenoid side chain. Coenzyme Q is also called ubiquinones because it is ubiquitous in all living system. Several ubiquinones are known, differing only in the number of isoprene units in their side chain.
The ubiquinone found in the mitochondria of animal tissues contains ten isoprene units and has been designated as CoQ10. The quinone ring is reversibly reducible to a hydroquinone, and so CoQ serves as the electron carrier between the flavin coenzymes and the cytochromes. It is in this step that the oxidized form of the flavin coenzyme, FAD, is regenerated.
A series of compounds called cytochromes were probably the first entities to be associated with electron transferring reactions. A number of these substances exist, and we have included five of them in Fig. 8.81. The cytochromes are conjugated protein enzymes. Their coenzymes are iron porphyrins, which resemble haeme, the pigment in haemoglobin and in myoglobin.
The various cytochromes differ with respect to:
(1) Their protein constituents,
(2) The manner in which the porphyrin is bound to the protein, and
(3) The substituents on the periphery of the porphyrin ring.
Such slight differences in structure bestow differences in reduction potential upon the different cytochromes. The characteristic feature of the cytochromes is the ability of their iron atoms to exist in either the Fe (II) (ferrous) or Fe (III) (ferric) form. Thus each cytochrome in its oxidised form, Fe (III), can accept one electron and be reduced to the Fe (II)-containing form.
This change in oxidation state is reversible, and the reduced form can donate its electron to the next cytochrome, and so on. Only the last cytochrome, cytochrome a3, has the ability to transfer electrons to molecular oxygen. (The combination of cyanide with the ferric ions of cytochrome a3 completely inhibits the transfer of electrons to molecular oxygen. This accounts for the extreme toxicity of cyanide to living organisms.)
The cytochromes are strictly electron carriers, the hydrogens of the reduced coenzyme Q10 are released as ions into the medium. (They are utilised in the last step in the reduction of oxygen to water.) Since the Fe (III)/Fe (II) system is only a one electron exchange, two cytochrome b molecules are necessary to complete the oxidation of coenzyme Q10H2:
Coenzyme Q10H2 → Coenzyme Q10 + 2H+ + 2e–
2Cyt b(Fe3+) + 2e– → 2Cyt b(Fe2+)
Then
Cyt b → Cyt c1 → Cyt c → Cyt a → Cyt a3
In the final step, two molecules of the terminal electron carrier cytochrome a3 pass their electrons to molecular oxygen, the ultimate election acceptor, It has been estimated that about 95% of the oxygen utilised by cells reacts in this single process:
2Cyt a3(Fe2+) → 2Cyt a3(Fe3+) + 2e–
½O2 + 2H+ + 2e– → H2O
Each intermediate compound in the respiratory chain is reduced by the addition of electrons and hydrogen ions in one reaction and is subsequently restored to its original form when it delivers the protons and electrons to the next compound. Thus, each pair of hydrogen atoms that is removed from the substrates of the Krebs cycle ultimately reduces one atom of oxygen.
Oxidative Phosphorylation:
The process whereby ATP is synthesised as a result of the operation of the respiratory chain is referred to as oxidative phosphorylation or respiratory chain phosphorylation. The details of the mechanism that links the formation of ATP to the operation of the respiratory chain are largely unknown.
It is known, however, that the energy required for the production of ATP results from the passage of a pair of electrons from one carrier to the next. The electron transport system can be thought to function as a biochemical battery; that is, energy is obtained from oxidation-reduction reactions.
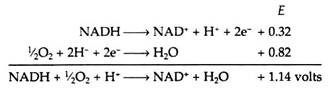
The sites on the respiratory chain at which the oxidative phosphorylations are believed to occur are shown in Fig. 8.79. From the data contained in Table 8.14, we can calculate the maximum amount of energy, E, that is made available when a single pair of electrons travels from NADH to oxygen along the chain.
The energy change for the reaction can be obtained by the use of the following equation.
Energy change = -nFΔE
where n = number of electrons transferred
F = Faraday’s constant (23,000 cal/volt equivalent)
Energy change = -(2)(23,000)(1.14)
= -52,000 calories
This value of 52,000 calories represents a considerable amount of energy. If it were released all at once, much of it would be dissipated as heat and it might prove damaging to the cell. Therefore, the respiratory chain serves as a device for delivering this energy in small increments to be used to phosphorylate ADP.
It has been experimentally observed that three molecules of ATP are formed for every molecule of NADH that is oxidized in the chain (but only two ATPs are formed if the primary acceptor is FAD, as in the case when succinic acid serves as a substrate). The net equation for the respiratory chain is
NADH + ½O2 + H+ + 3ADP + 3P1 → NAD+ + H2O + 3ATP
Almost half of the energy released in the electron transport process is conserved in the formation of high energy phosphate bonds:
Energy conserved by respiratory chain = (3)(7300)(100)/(52,000)
= 42%
Energy Yield of Carbohydrate Metabolism:
It is now possible to summarise the energy conserved (in ATT production) from the complete oxidation of one molecule of glucose. It must be recalled that, under aerobic conditions, the glycolytic sequence terminates with the formation of two molecules of pyruvic acid. Two molecules of ATP are obtained from substrate level phosphorylation. In addition, since the pyruvic acid is not reduced to lactic acid, there are two molecules of NADH (from step 6) that remain in the cytoplasm.
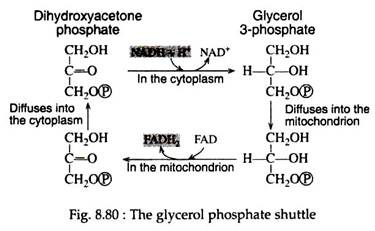
We know that NAD+ must be regenerated from NADH for glycolysis to continue. The problem, however, is that NADH cannot pass across the membrane of the mitochondrion to be oxidised by the respiratory chain. A solution is achieved by a shuttle process involving glycerol 3- phosphate and dihydroxyacetone phosphate. These compounds can penetrate the mitochondrial barrier.
The first step in this shuttle (Fig. 8.80) is the reduction of dihydroxyacetone phosphate by NADH to form glycerol 3-phosphate and to regenerate the NAD+. Glycerol 3-phosphate then enters the mitochondrion where it is re-oxidised to dihydroxyacetone phosphate, this time by a dehydrogenase that contains the coenzyme FAD instead of NAD+.
The dihydroxyacetone phosphate exits the mitochondrion and returns to the cytoplasm to complete the shuttle. The reduced form of the flavin coenzyme FADH2 is re-oxidised by passing its electrons to coenzyme Q10 in the respiratory chain.
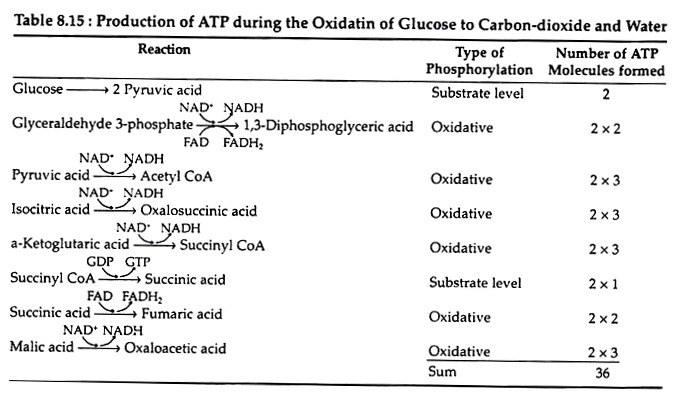
As a result, two molecules of ATP (instead of three) are formed every time a cytoplasmic NADH molecule is re-oxidised via the glycerol phosphate shuttle and the respiratory chain. The aerobic continuation of glycolysis yields total of 15 molecules of ATP from each molecule of pyruvic acid or 30 molecules of ATP per molecule of glucose. Table 8.15 lists the various reactions that result in ATP synthesis:
C6H12O6 + 6O2 + 36ADP + 36Pi → 6CO2 + 6HZO + 36ATP
The above is the net equation for the oxidation of one mole of glucose in aerobic cells. The energy released (6,86,000 calories) is coupled to the synthesis of 36 moles of ATP from ADP and inorganic phosphate. Assuming that 7,300 calories is required for the synthesis of each mole of ATP, then 36 × 7,300 or 263,000 calories is conserved by the cell. The efficiency of conservations is thus:
(2,63,000)(100)/(6,86,000) = 38%
This recovery of energy compares favorably with the efficiency of any man-made machine, and it represents a remarkable achievement on the part of the living organism.
Lipid Metabolism:
In animals all the energy, required for body activities is supplied by the oxidation of carbohydrates and lipids. Carbohydrates act as instant source of energy, while lipid as reserve source. The amount of energy stored in fats far exceeds that stored in the form of glycogen. However, lipids are not dietary necessities; an organism can survive on a lipid-free diet if carbohydrates and proteins are supplied regularly.
Certain lipids, known as essential fatty acids, are required for normal growth and development. These lipids cannot be synthesized by the animals in their body. Some of the important essential fatty acids are linoleic acid, linolenic acid and arachidonic acids. These are unsaturated fatty acids. Essential fatty acids are necessary for maintaining the structure of cell membrane and for the efficient transport and metabolism of cholesterol.
Absorption of Lipids:
In the intestine the lipids are hydrolysed and absorbed in the form of monoacylglycerols, diacylglycerols and fatty acids. After absorption through the intestinal epithelium, they are readily re-synthesised into triacylglycerols or phospholipids. The acylglycerols then become associated with proteins and are transported into the blood stream via the lymphatic system.
Some of the fat is carried to the liver. The liver is the responsible organ for maintaining the proper physiological lipoprotein concentration. Lipids are also transported to different organs for various functions. The fate of lipids in animal body is outlined below (Fig. 8.85).
Oxidation of Fatty Acids:
Triacylglycerols are cleaned to fatty acids and glycerols by lipases before they are used for production of energy. The glycerol is converted to a carbohydrate derivative and is metabolised via the Embden-Meyerhof pathway. The fatty acids, which contain the bulk of the energy of the lipids, are broken down in a series of sequential reaction accompanied by the gradual release of utilizable energy.
Some of these reactions are oxidative and require the same coenzymes (NAD+, FAD) as those which take part in the oxidation of carbohydrates. The enzymes involved in fatty acid catabolism are localised in the mitochondria along with the enzymes of the Krebs cycle, respiratory chain, and oxidative phosphorylation. This factor (the localisation within the mitochondion) is of the utmost importance since it provides for the efficient utilisation of the energy stored in the fatty acid molecules.
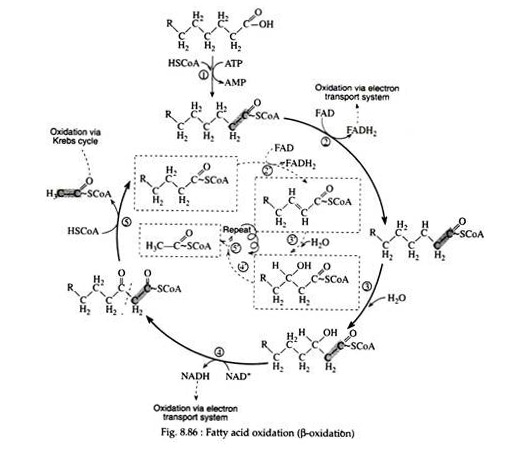
In the beginning of the twentieth century, the German biochemist Franz Knoop showed that the breakdown of fatty acids occurs in a stepwise fashion with the removal of two carbon atoms at a time. The reaction scheme is shown in Fig. 8.86.
Step 1:
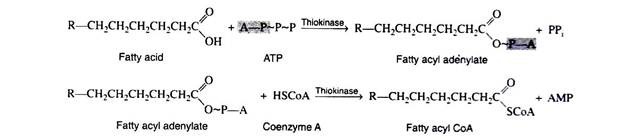
Fatty acids, like carbohydrates and amino acids, are relatively inert and must first be activated by conversion to an energy-rich fatty acid derivative of coenzyme A (called fatty acyl CoA). This activation process is a two-step reaction that is catalyzed by the enzyme acyl CoA synthetase (also called thiokinase).
For each molecule of fatty acid that is activated, one molecule of coenzyme A and one molecule of ATP are used. First the fatty acid reacts with ATP to form a fatty acyl adenylate. Then the sulfhydryl group of coenzyme A attacks the acyl adenylate to yield fatty acyl CoA and AMP.
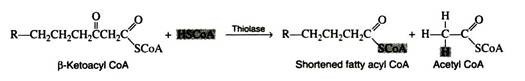
The fatty acyl CoA now enters into a sequence of reactions known as β-oxidation (since the carbon beta to the carboxyl group undergoes successive oxidation), or the fatty acid spiral (because the fatty acid goes through the cycle again and again until it is finally degraded to acetyl CoA molecules).
The reactions involved in steps 2 through 4, dehydrogenation, hydration, and dehydrogenation, are analogous to those involved in the conversion of succinic acid to oxaloacetic acid (succinic → fumaric → malic → oxaloacetic).
The functional group at the beta carbon can be visualised as undergoing the following changes: alkane → alkene → secondary alcohol → ketone. The net effect is the introduction of a keto group beta to a carboxyl group.
Step 2:
This first oxidation reaction is catalyzed by the enzyme acyl dehydrogenase. The coenzyme FAD accepts two hydrogen atoms from adjacent carbons, one from the a-carbon and one from the β-carbon. The enzyme is stereospecific in that only the Trans alkene is obtained.
Each molecule of FADH2 that is re-oxidised via the electron transport chain supplies energy to form two molecules of ATP.
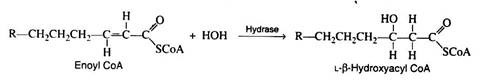
Step 3:
Again, as in the Krebs cycle, only the L-isomer is formed when the stereospecific enzyme enoyl CoA hydrase adds water across the trans-double bond:
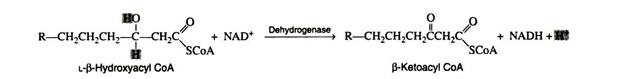
Step 4:
The second oxidation reaction is catalysed by L-β-hydroxyacyl dehydrogenase, which exhibits an absolute stereospeoificity for the L-isomer. Here the coenzyme NAD+ is the hydrogen acceptor:
The re-oxidation of NADH and the transport of hydrogen ions and electrons through the respiratory chain furnish three molecules of ATP.
Step 5:
The final reaction is a cleavage of the β-ketoacyl CoA by a molecule of coenzyme A. The products of the reaction are acetyl CoA and a fatty acyl CoA whose chain length is shortened by two carbon atoms. The enzyme is β-ketothiolase.
The newly formed fatty acyl CoA can then be degraded further by repetition of steps 2 through 5; a molecule of acetyl CoA is liberated at each turn of the spiral. Normally, the spiral is repeated as many times as is necessary to break down a fatty acid containing an even number of carbon atoms, n, into n/2 molecules of acetyl CoA.
It should be noted that no further addition of ATP is necessary since the shortened fatty acids already contain the thiol ester. One molecule of ATP is sufficient to activate any fatty acid regardless of the number of carbon atoms in its hydrocarbon chain.
The unsaturated fatty acids are also incorporated into the β-oxidation sequence. Two ancillary enzymes are required to convert their degradation pro-ducts into normal substrates of the fatty acid spiral.
The acetyl CoA formed in fatty acid oxidation may enter the Krebs cycle or may be utilised in a variety of synthetic reactions (e.g. formation of triacylglycerols, phospholipids, cholesterol). The overall equation for one turn of the fatty acid degradation spiral is:
Energy yield in a Fatty acid Oxidation:
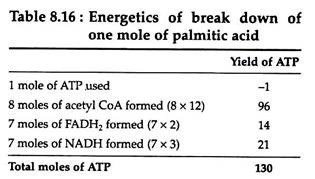
The combustion of one mole of palmitic acid releases a considerable amount of energy, and the precise amount can be determined by conducting the experiment in a calorimeter:
C16H32O2 + 23O2 → 16CO2 + 16H2O + 2,338,000 calories
The amount of energy that is made available to the cell and conserved in the form of ATP is readily calculable. The breakdown by an organism of one mole of palmitic acid necessitates the utilization of one mole of ATP, and eight mole of acetyl CoA are formed. You can recall that each moles of acetyl CoA metabolised by the Krebs cycle yields twelve moles of ATP. Each turn of the fatty acid spiral produces one mole of NADH and one mole of FADH2.
Re-oxidation of these compounds by the respiratory chain yields three and two moles of ATP, respectively. The complete degradation of palmitic acid requires seven turns of the spiral, and a total of seven moles of FADH2 and NADH is, therefore, formed. The energy calculations may be summarised as:
The percentage of available energy that can theoretically be conserved by the cell in the form of ATP is
(130)(7300)(100)/2,338,000 = 41%
The efficiency with which fatty acids are metabolised is seen to be comparable to that of the carbohydrate metabolism system.
Ketosis:
In general, all of the acetyl CoA formed in the breakdown of fatty acids is utilised by the body. Any abnormal physiological condition that causes cells to metabolize their stored fats at an accelerated rate will result in the production of more acetyl CoA than can be oxidised by the Krebs cycle or utilised in synthetic reactions.
Such a condition generally occurs in conjunction with an impairment of carbohydrate metabolism, for example, fasting, diabetes mellitus, or certain types of cellular poisoning. In these cases, very little carbohydrate is available to the cell, and, therefore, the production of oxaloacetic acid is drastically reduced. Recall that acetyl CoA enters the Krebs cycle by condensing with oxaloacetic acid.
If this latter compound is not present in sufficient quantity, acetyl CoA will accumulate. Furthermore, in the absence of carbohydrate metabolism, the rate of fatty acid oxidation increases to meet the energy demands of the cell, and the build-up of acetyl CoA increases accordingly.
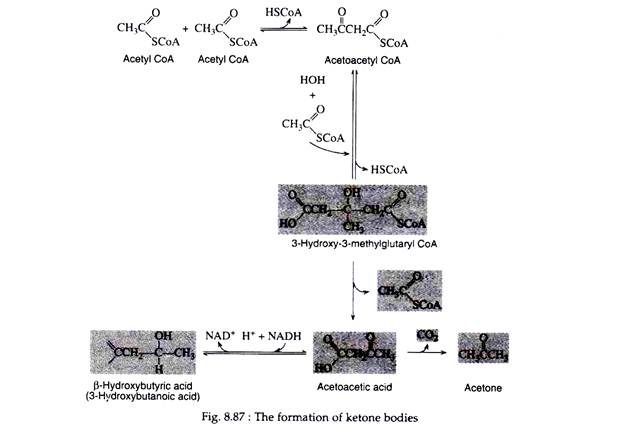
When the concentration of acetyl CoA in the cell reaches a certain level, a reversal of the last step of the fatty acid spiral occurs to produce aceto-acetyl CoA. The acetoacetyl CoA reacts with another molecule of acetyl CoA and with water to form 3-hydroxy-3 methylglutaryl CoA, which is then cleaved to aceto-acetic acid and acetyl coenzyme A (Fig. 8.87).
Since some aceto-acetic acid is normally produced in fatty acid metabolism, cells possess a mechanism for its oxidation. When, however, the rate of formation of acetoacetic acid exceeds that of its disposal the excess amount may either be reduced to form β- hydroxybutyric acid or be decarboxylated to CO2 and acetone.
Acetoacetic acid, β-hydroxyburyric acid, and acetone are collectively referred to as ketone bodies, and their accumulation in the blood (at concentrations greater than 1 mg/ 100ml) and urine leads to a condition known as ketosis. A general scheme for the formation of ketone bodies is shown in Fig. 8.86. Since two of the three ketone bodies are acids, their presence in the blood in excessive amounts causes a marked decrease in its pH.
This decrease in the pH of the blood leads to a more serious condition known as acidosis. Acidosis results in interference with the transport of oxygen by the haemoglobin molecule and a fatal coma may result. Any treatment that promotes the utilisation of carbohydrates (for example, an injection of insulin) can alleviate both ketosis and acidosis.
Glycerol Metabolism:
One molecule of glycerol is obtained from each molecule of triacylglycerol or phospholipid that is hydrolysed. Glycerol is readily incorporated into the scheme of carbohydrate metabolism by conversion to dihydroxyacetone phosphate, a key intermediate in the glycolytic pathway.
This conversion includes the phosphorylation of a primary hydroxyl group followed by the oxidation of a secondary hydroxyl group to a ketone. This last reaction is readily reversed whenever glycerol phosphate is required for the synthesis of triacylglycerols. 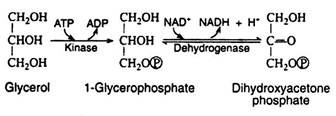
Synthesis of Fatty Acids:
Both plant and animal organisms can convert their excess carbohydrates into lipids. Animal cells are unable to accomplish the reverse process, whereas plant seeds rapidly convert their fat deposits to sucrose upon germination. Apparently animal tissues lack essential enzymes that are present in the seeds.
We have seen that acetyl CoA is a common intermediate of both fatty acid, and carbohydrate degradation; therefore, it is logical to assume that this compound is the starting material for the synthesis of fatty acids. The original hypothesis was that the reactions of the fatty acid spiral were reversible, and that fatty acid biosynthesis proceeded by a simple reversal of the degradation reactions.
However, in the early 1950s, the enzymes of the β-oxidation sequence were isolated, purified, and shown to be incapable of catalysing the biosynthesis of fatty acids from acetyl CoA. These crucial experiments launched many investigators on an active search for a suitable pathway for the synthetic process.
Thanks largely to the work of Feodor Lynen (Nobel Prize for Medicine, 1964), we now know that the enzymes which catalyse fatty acid synthesis are found in the cytoplasm rather than in the mitochondria where the enzymes for fatty acid degradation are located. Furthermore, all of these enzymes are associated in a single enzyme complex known as fatty acid synthetase.
The various intermediates produced during the synthetic process are covalently bound to the synthetase complex by sulfhydryl group (-SH). No free intermediates are released, and the correct sequence of reactions is governed by the position of each enzyme in the complex.
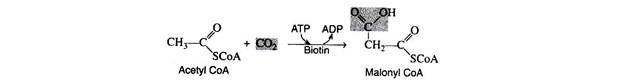
In 1959, several researchers observed that the biosynthesis of fatty acids requires ATP, CO2, and the coenzyme biotin. It was postulated that the first step is the carboxylation of acetyl CoA to malonyl CoA in the presence of the enzyme acetyl CoA carboxylase.
The mechanism of the reaction is quite complex. Present evidence indicates that carbon dioxide covalently a complex with biotin and the CO2 is then transferred to acetyl CoA in a subsequent step. The hydrolysis of ATP provides the energy for the formation of the CO2– biotin complex.
CO2 + Biotin + ATP → CO2-biotin + ADP + Pi
CO2-biotin + Acetyl CoA → Malonyl CoA + Biotin
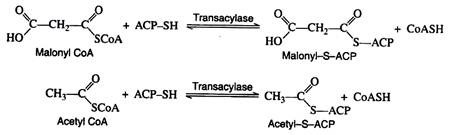
A relatively low molar mass protein (~10,000) has been identified as a key component in fatty acid biosynthesis. This protein, called acyl carrier protein (ACP-SH), contains a sulfhydryl group that covalently bonds to the acyl intermediates.
The function of ACP-SH in fatty acid biosynthesis is to serve as a sort of anchor to which the acyl intermediates are attached. This process is described as trans acylation—an acyl group is transferred from a coenzyme A thioester to the sulfhydryl group of the acyl carrier protein.
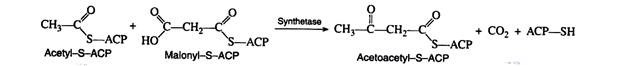
Malonyl-S-ACP and acetyl-S-ACP then combine to form acetoacetyl-S-ACP. Notice that the carbon dioxide released in this reaction is the same CO2 that was required for the formation of malonyl CoA from acetyl CoA. Carbon dioxide can thus be considered to play a catalytic role in fatty acid biosynthesis. It is utilised in one step and regenerated in a later step.
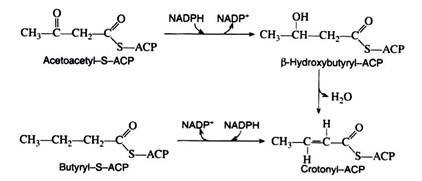
The next three reactions in the anabolic sequence are reduction, dehydration, and a second reduction. These processes are essentially the reverse of the catabolic reactions, except that here both reduction reactions are catalysed by NADPH rather than by NADH and FADH2
The butyryl-S-ACP then reacts with another molecule of malonyl-S-ACP, carbon dioxide is given off, and the entire series of reactions is repeated. In this manner, the hydrocarbon chain of a given fatty acid synthesise two carbon units at a time. This explains why the naturally occurring fatty acids contain an even number of carbon atoms.
We have said that plants are able to synthesize fatty acids containing several double bonds. Animals are only capable of introducing one double bonds into a fatty acid. They can convert stearic acid to oleic acid but lack the appropriate enzymes to carry out further dehydrogenations. The overall equation for the synthesis of palmitic acid is written as:
It should be noted that the existence of these separate pathways for the synthesis and degradation of fatty acids is analogous to the situation that exists for the anabolism and catabolism of glycogen. Again, the presence of separate pathways permits the two operations to proceed independently of each other. The rates of oxidation and biosynthesis are only governed by the general metabolic state of the organism.
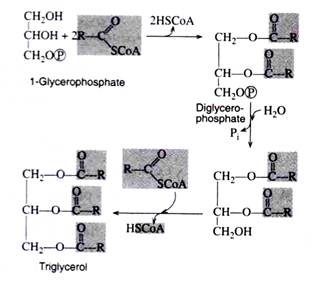
Biosynthesis of Triacylglycerols:
The coenzyme A derivatives of the fatty acids condense with glycerophosphate to form triacylglycerols. The composition of the resulting simple lipids depends upon which fatty acids and which specific enzymes participate in the synthetic reactions.
Protein Metabolism:
Dietary protein of animals cannot normally be absorbed across intestinal membranes. These are digested in the gastrointestinal tract into the amino acids by the proteolytic enzymes. These amino acids are actively transported (an energy-requiring process) across the intestinal wall into the portal circulation and are carried to the liver. The liver is the principal organ responsible for the degradation and synthesis of amino acids.
It is also the site of synthesis of several blood proteins, e.g., albumins, globulins, fibrinogen, prothrombin, etc. The amino acids synthesized in the liver and the amino acids obtained from protein digestion, along with the amino acids derived from the turnover of tissue proteins, are transported by the blood to all the tissues of the body. Therefore, protein metabolism is equivalent to amino acid metabolism.
Amino acids supply nitrogen to an organism for proper maintenance and growth of the body. On the other hand, nitrogen is lost in continuous degradation of tissue protein and in the excretion of certain nitrogen containing waste materials. Under normal conditions, an individual’s intake of dietary nitrogen is equal to the amount of nitrogen in the excrement—such a condition is referred to as nitrogen balance or nitrogen equilibrium.
Organisms are said to be in positive nitrogen balance (intake exceeds excretions) whenever, tissue is being synthesised, as, for example, during growth periods.
Negative nitrogen balance results from:
(i) An inadequate intake of protein (e.g. fasting),
(ii) An accelerated catabolism of tissue protein (e.g., fever, infections, wasting diseases), or
(iii) A diet that lacks, or is deficient in, any one of the essential amino acids.
Animals are capable to synthesise only about half of their naturally occurring amino acids; the remainder are supplied through the diet. An essential or indispensible amino acid (Table 8.17) is one that cannot be synthesised by an organism from the substrates ordinarily present in its diet, at a rate rapid enough to meet the demands of natural growth and repair.
The average individual requires about 50 gms of proteins in daily diet. Prolonged deprivation of protein leads to exhaustion of all carbohydrate and fat reserves; the organism is ultimately left with no source of energy except its own tissue proteins.
The Nigerian civil war (1967-68) made the world brutally aware of the serious condition of protein deprivation called kwashiorkor, a disease resulting in extreme emaciation, bloated abdomen, discolouration of the hair, and eventual death. This disease is prevalent in Latin America, Asia and Africa and can be best treated by the administration of animal protein.
Metabolism of Amino Acids:
Most of the amino acids in the metabolic pool are utilised for the synthesis of any of the myriad of proteins necessary to the living organism. This is the major function of amino acids in the body. About 75% of the metabolized amino acids in the normal adult are used for this purpose.
This is necessary because of the constant destruction of body proteins by wear and tear. It should be emphasised, however, that amino acids also play an essential role in the metabolism of all nitrogenous compounds. In addition, amino acids may be catabolized to yield glycogen, fat, or energy, or they may participate in a specific biochemical pathway.
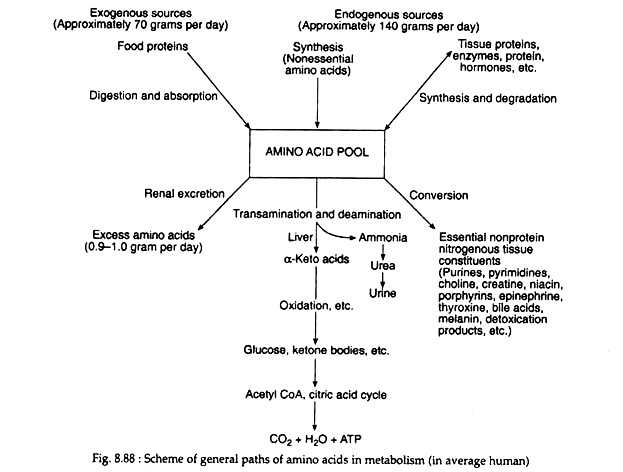
Generally, the first step in the breakdown of amino acids is the separation of the amino group from the carbon skeleton. The amino groups are then incorporated into almost all the other nonprotein nitrogen containing compounds, for example, hormones, nucleic acids, porphyrins, and creatine (Fig. 8.88). A discussion of these specific metabolic pathways is beyond the scope of this text.
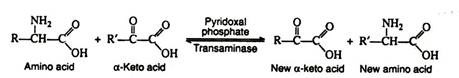
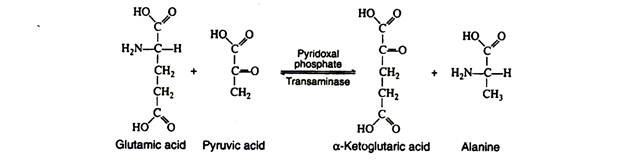
a. Transamination:
In this reaction, the amino group is transferred from an amino acid to an α-keto acid, resulting in the formation of a new keto acid and a new amino acid. Practically all of the naturally occurring amino acids have been found to undergo this reaction.
It is probable that there are a large number of transaminases, each one catalysing a reaction between a α-amino acid and some α-keto acid. The transaminases are widely distributed in nature, and are known to function in conjunction with the coenzyme pyridoxal phosphate. It transfers the amino group to the appropriate α-keto acid (usually pyruvic acid, oxaloacetic acid, or α-keto-glutaric acid).
The production of α-keto acids by this process yields molecules that can enter the glycolytic pathway or the Krebs cycle. A single reaction is necessary for the conversion of alanine, glutamic acid and aspartic acid to pyruvic acid α-ketoglutaric acid and oxalo-acetic acid, respectively. Additional reactions are necessary to form metabolic intermediates from the other amino acids.
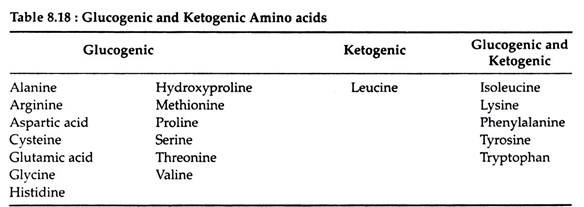
It is especially significant that these reactions are reversible, since this reversibility links protein metabolism with the metabolism of carbohydrates and lipids. Those amino acids that can form any of the metabolites of carbohydrate metabolism can be converted to glucose or glycogen (or oxidised to CO2, H2O and energy) and are referred to as glucogenic.
Amino acids that give rise to acetyl CoA or acetoacetyl CoA are said to be ketogenic. Certain amino acids fall into both categories; leucine is the only amino acid that is exclusively ketogenic. Table 8.18 represents the classification of amino acids with regard to the metabolic fate of their carbon skeletons.
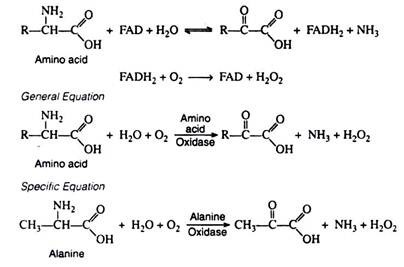
b. Oxidative Deamination:
Oxidative deamination effects the replacement of the amino group by oxygen. This reaction is catalyzed by amino acid oxidase, an enzyme containing the oxidising coenzyme FAD. The reduced coenzyme is re-oxidised by molecular oxygen (and not by the respiratory chain) to form H2O2.
Once again, the α-keto acids that are formed can be incorporated into the schemes of carbohydrate or lipid metabolism. The other products of the reaction, NH3 and H2O2, are highly toxic and must be removed from the body. Ammonia is converted to urea and excreted in the urine. Under the influence of catalase, hydrogen peroxide is decomposed to water and oxygen. Catalase is extremely efficient: a single enzyme molecule catalyzes the decomposition of about 5 × 104 molecules of H2O2 per second:
H2O2 catalase→ H2O + ½O2
Although amino acid oxidases are widely distributed in nature, they are limited to the kidney and the liver of mammals. Furthermore, their activity in these organs is extremely low, and removal of amino groups by oxidases is of less physiological significance than removal by transaminases.
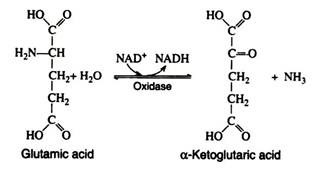
There are some specific amino acid oxidases that require pyridine nucleotides: in these cases, the reduced coenzymes (NADH and NADPH) are oxidised by way of the respiratory chain. The most important specific oxidase is glutamic acid oxidase. It has been crystallized from beef liver and shown to contain divalent zinc ions.
It catalyses the following reaction:
The reverse of this reaction provides a means for the synthesis of amino acids from ammonia and an intermediate of carbohydrate metabolism. The amino group in the glutamic acid can be passed on through transamination reactions, producing all the other cellular amino acids, provided the appropriate α-keto acids are available.
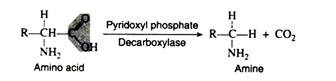
c. Decarboxylation:
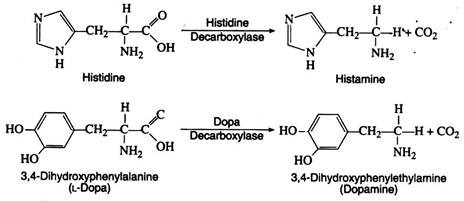
Several amino acid decarboxylases exist, which eliminate carbon dioxide from amino acids to form primary amines. Pyridoxyl phosphate is the necessary coenzyme:
These enzymes are found primarily in microorganisms but they are also found in some animal tissues. Intestinal bacteria are responsible for amino acid decarboxylation, and the foul smell of the faeces is due in part to the resulting amines. Some of the amines produced as a result of decarboxylation have important physiological effects.
Thus histamine, formed by decarboxylation of histidine, causes a decrease in blood pressure and can stimulate gastric secretions. Another decarboxylase catalyses the decarboxylation of 3, 4-dihydroxyphenylalanine (L-dopa) to form dopamine. A deficiency of dopamine in the brain cells is a primary cause of Parkinson’s disease, a disorder of the central nervous system.
Dopamine itself cannot be administered because it does not pass across the blood-brain barrier. A major breakthrough in the treatment of Parkinson’s disease has been the use of L-dopa. Large dose of L-dopa are administered orally; the drug is able to pass from the digestive system into the blood and then cross the blood-brain barrier. In the brain cells, L-dopa is converted into the necessary compound, dopamine.
Storage of Nitrogen:
In contrast to carbohydrates and lipids, proteins are not stored to an appreciable extent in living organisms. Small amounts of amino acids are excreted by humans, but this is essentially wasteful process since amino acids contain utilizable energy.
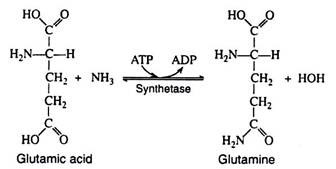
Accordingly, most excess amino acids are deaminated; the resulting carbon skeleton is oxidized to produce energy or is stored as glycogen or fat. Most of the ammonia that is liberated is converted to urea. A relatively small proportion combines with glutamic acid in the presence of glutamic acid synthetase and ATP to form glutamine (the amide of glutamic acid).
Glutamine is present in many tissues and in the blood and serves as a storage form of nitrogen. The amino group can be donated in specific reactions to appropriate acceptor molecules to form a variety of nitrogenous compounds (purines, pyrimidines, NAD+). Asparagine, the amide of aspartic acid, is a common constituent of many plant proteins and is probably the storage form of nitrogen in plants.