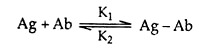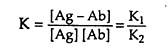The below mentioned article provides notes on Antigen Antibody Interaction.
Structural and Chemical Basis of Antigen Binding:
The antigen binding sites of most antibodies are planar surfaces that can accommodate conformational epitopes of macromolecules. The recognition of antigen by antibody involves non-covalent, reversible binding. Because the strength of each of such interaction is weak, a large number of such interactions are required to form a strong Ag- Ab interaction.
Moreover, such non-covalent interactions operate over a very small distance, generally about 1 × 10−7 mm or a little less. Consequently, a strong Ag-Ab interaction requires a very close fit between the antigen and antibody. Such fits require a high degree of complementarity between the epitopes and antigen-binding sites of antibody molecule.
ADVERTISEMENTS:
Antibody Affinity and Antibody Avidity:
The strength of the binding between a single antigen binding site of an antibody and an epitope of an antigen is called the affinity of the antibody. Serum from an immunized individual will contain a mixture of antibodies with different affinities for the antigen, depending primarily on the amino acid sequences of the CDRs.
Low affinity-antibodies bind antigen weakly and tend to dissociate readily, whereas high affinity antibodies bind antigen more tightly and remain bound longer.
A single antibody may attach to a single multivalent antigen by more than one binding site. For IgG or IgE, this attachment can involve, at most, two binding sites, one on each Fab. For pentameric IgM, however, a single antibody may bind at up to 10 different sites.
ADVERTISEMENTS:
When complex antigens containing multiple and repeating antigenic determinants are mixed with antibodies containing multiple binding sites, the interaction of an antibody molecule with an antigen molecule at one site will increase the probability of reaction between those two molecules at a second site.
The strength of such multiple interactions between a multivalent antibody and antigen is called the avidity and it is much greater than the affinity of any one antigen-binding site. High avidity can compensate for low affinity. For example, secreted pentameric IgM often has a lower affinity than IgG, but the high avidity of IgM, resulting from its higher valency, enables it to bind antigen effectively.
The association between a binding site on an antibody (Ab) with a monovalent antigen (Ag) can be described by the equation
where Ag-Ab is the bound complex. The forward and reverse binding reactions are characterised by K1 and K2 rate constants, respectively. The affinity of the antibody’s binding site can be measured by the ratio of complexed to free reactants at equilibrium. Thus, an affinity constant K is defined by
where the brackets denote concentration. K can be determined by measuring the concentration of free antigen needed to fill half the binding sites of an antibody. Typical values of K are 105 – 1011 litres/mole. At this point [Ag – Ab] must equal [Ab], and the above formula resolves to
K = 1/[Ag]
Many proteins may have weak affinities for other proteins; consequently we need to define an affinity below which binding is considered to be nonspecific and above which binding is considered to be a specific antibody-antigen reaction. From experience, any K less than 104 liters/mole is considered nonspecific.
But antibody-antigen reactions are not necessarily the simple bimolecular events treated in the above equation. Such a treatment is appropriate for an antigen with one potential epitope for reaction and for a homogeneous antibody population, even though there are two binding sites per antibody molecules.
For instance, if the two antibody sites can react with the same antigen molecule, the second site will react much faster than the first because the antigen molecule will already be very close and the apparent affinity will be greatly increased.
A further complication is that natural antibody populations can react with a multitude of epitopes on antigens, with a variety of affinities. Then avidity is used to describe the apparent affinity of an antibody population reacting with an antigen. Avidity is measured by half-saturation of antibody, but such measure has no simple mathematical meaning.
Antigen-Antibody Reaction:
The interaction between antigen and antibody occurs in two stages:
ADVERTISEMENTS:
1. Primary stage:
In the primary or initial stage, non-covalent binding between the antigen and antibody produces small and soluble antigen-antibody complexes as a result of primary union. This primary reaction has no visible effect and the reaction is rapid. However, microscopic localisation of antibody can be done on a component of particular microorganism if the antiserum is labelled with fluorescent dye.
2. Secondary stage:
The primary union in most cases, but not all, is followed by changes in their three dimensional form. Therefore, antigen-antibody complexes soon develop into insoluble crystal-lattices with physically demonstrable changes like precipitation or agglutination due to secondary effect.
1. Precipitation Reaction:
When a soluble antigen interacts with its specific antibody to form a lattice that eventually develops into a visible precipitate, the reaction is referred to as precipitation. Antibodies that precipitate soluble antigens are called precipitins.
In precipitation reaction, formation of soluble Ag-Ab complex occurs within minutes, but formation of the visible precipitate occurs more slowly and often takes a day or two to reach completion. When the precipitate remains suspended instead of sedimenting, the reaction is called flocculation. Precipitation reaction may occur in liquid medium or in semi-solid medium like agar gels, agarose or polyacrylamide.
Criteria for Precipitation:
i. Precipitation reaction depends on the valency of both antigen and antibody molecules.
ii. For this reaction, antibody must be bivalent; a precipitate will not form with monovalent Fab fragment.
iii. The antigen in this case must be either bivalent or polyvalent. That means, it must have at least two copies of the same epitope or have different epitopes that react with different antibodies present in polyclonal antisera.
Mechanism of precipitation:
According to Marrack’s (1934) view on lattice hypothesis, multivalent antigens combine with bivalent antibodies in optimal proportions. Following the formation of initial combination of Ag and Ab molecules, free antigen binding sites in antibody and free epitopes in antigen, enable the complexes to link up to the formation of large lattice in the zone of equivalence.
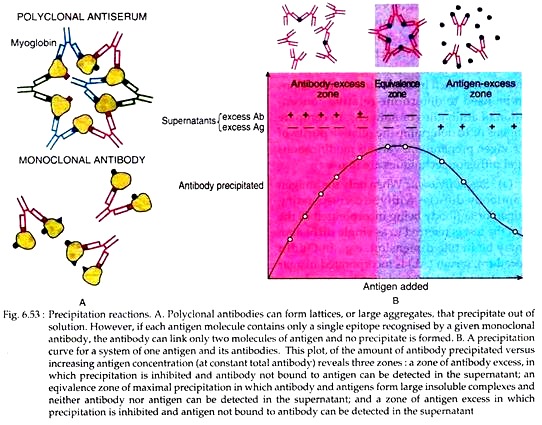
The antigen and antibody molecules interact in such a way that large three dimensional lattice structures through Fc-Fc are formed. Such lattice consists of alternating Ag and Ab molecules (Fig. 6.53). When the size of lattices exceeds critical volume, they settle out of the solution spontaneously.
In the region of antibody excess, unreacted antibody is found in the supernatant along with small, soluble complexes consisting of multiple molecules of antibody bound to a single molecule of antigen. In the region of antigen excess, unreacted antigen can be detected and small, soluble complexes consisting of one or two molecules of antigen bound to a single molecule of antibody could be observed.
In either case, extensive lattices cannot be formed and precipitation is inhibited. But, when the ratio of antibody to antigen is optimal, large multi-molecular lattice is formed at equivalence. Such reaction can be shown by a precipitation curve for a system of one antigen and its antibody (Fig. 6.53).
Techniques of Precipitation:
There are three principal types of precipitation techniques:
(1) Simple precipitation test,
(2) Gel diffusion test,
(3) Immunoelectrophoresis.
1. Simple precipitation test:
It is of two types:
(a) Ring test, and
(b) Slide test.
(a) Ring test:
In this test a solution of test antigen is slowly layered on to a solution of antisera in a narrow test tube. Following a period of incubation, a white ring of precipitate develops at the junction of two solutions. This test is applied in tests for C reactive protein; Lancefield method of grouping beta-haemolytic streptococci.
(b) Slide test:
This test is also called flocculation test and is done both on slide and in tube. In case of slide test, a drop of antigen is added to a drop of de-complemented antiserum, mixed well and shaken for a few minutes. Visible precipitate appears in positive cases.
It is used in VDRL test for syphilis. In case of tube flocculation test, serial dilutions of antigen are taken in test tubes to which fixed amount of antisera are added. However, such test is not very sensitive and, thus, has been discarded now a days.
2. Gel diffusion test:
Precipitation test can be done in an agar gel. When antigen and antibody diffuse toward one another in agar or when antibody is incorporated into the agar and antigen diffuses into the antibody-containing matrix, a visible line or band of precipitation will form.
Such band or line can be stained for better viewing as well as for preservation. Such immune-diffusion reactions can be used to determine relative concentration of antibodies or antigens, to compare antigens or to determine the relative purity of an antigen preparation.
Various modifications of gel diffusion techniques are in use:
(a) Single diffusion:
When only the antigen or antibody diffuses with the corresponding antigen or antibody being incorporated in the agar, the test is referred to as single diffusion. It may be in one dimension, e.g., in Oudin procedure, serum (Ab) is incorporated in agar gel in a test tube and Ag solution is layered over it. Antigen diffuses downward forming a line of precipitation which can be viewed easily.
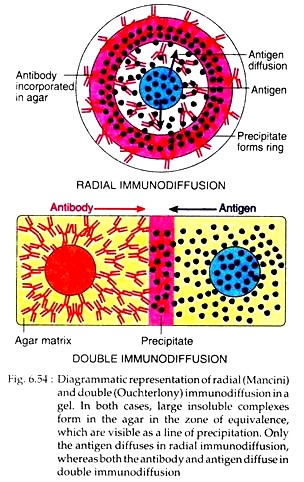
In radial immunodiffusion, antibody is incorporated in agar gel placed on petridish or slide. Antigen is kept in well cut out into gel. As the antigen diffuses, concentric bands of precipitin form around the well (Fig. 6.54). By comparing the area of the precipitation band with a standard curve, the concentration of the antigen can be calculated.
(b) Double diffusion:
When both antigen and antibody diffuse through the agar gel, it is called double diffusion. In such technique, where both Ag and Ag meet each other in optimal proportion, a visible line of precipitate is formed. Several varieties of double diffusion are available.
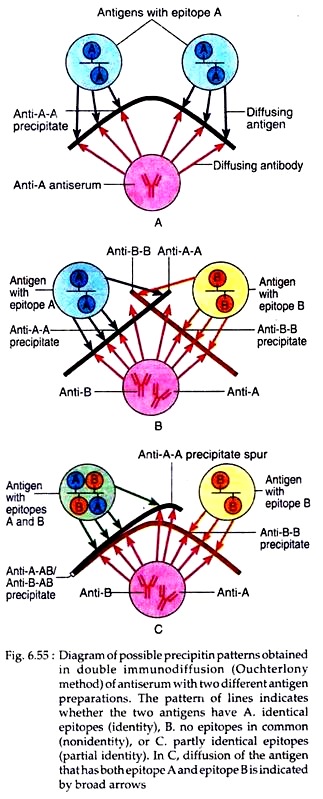
In Ouchterlony method, wells are cut on agar gel on a slide using a template. Antiserum is placed in the central well and different antigens in the surrounding well. Both these molecules are allowed to diffuse for some time. As both antigen and antibody diffuse radially from wells toward each other, a concentration gradient is established and at the zone of equivalence, visible line of precipitin is formed (Fig. 6.54).
The pattern of precipitin line that is formed will indicate whether antigens placed in adjacent wells have similar epitopes or not. If two antigens placed in adjacent wells share identical epitopes, the line of precipitin grow toward each other and fuse to give a single curved line of identity (Fig. 6.55A).
When two antigens are unrelated, having no common epitopes, the antiserum form independent precipitin line with each antigen and the two lines cross. This is referred to as nonidentity (Fig. 6.55B). If two antigens share some common epitopes, and some non-identical epitopes, then the antiserum forms a line of identity with the common epitopes and a curved spur with the non-identical. This is referred to as partial identity (Fig. 6.55C). This method was used in diagnosis of small pox and can be used to determine the purity of antigens.
3. Immunoelectrophoresis:
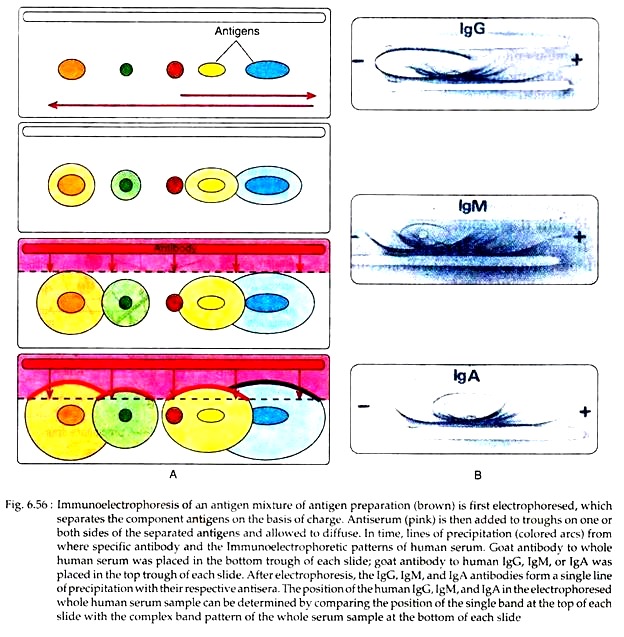
Immunoelectrophoresis combines electrophoresis with immunodiffusion. On the basis of the difference in surface charges between the different protein molecules of antigens, several antigens can be separated from a mixture by electrophoresis in agar gel. This test is performed in two stages.
In stage 1, antigen mixture is placed in a well cut into agar gel on slide or plate and electric current is passed through the agar for some time. According to the charges, the antigens migrate in the electric field and get separated from each other.
In stage 2, a trough is cut in the gel parallel to the direction of migration of antigen and filled with antibody, and allowed to diffuse for an appropriate time. As the antibody and antigen diffuse toward each other, a series of precipitin arcs are formed (Fig. 6.56) where the antibodies encounter the antigen in optimal proportions.
This technique is useful for determination of presence or absence of serum proteins (e.g., albumin, immunoglobulin etc.) and detection of unusual proteins such as human myeloma protein. However, it is strictly a qualitative technique that can detect relatively high antibody concentrations.
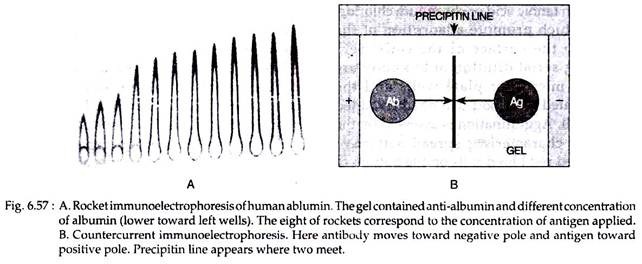
In rocket Immunoelectrophoresis, the antigen is electrophoresed through an agar gel containing antibody. This is a qualitative method. Antigen antibody complexes precipitate to form cone like structure (looking like a rocket); the length of which corresponds to the concentration of antigen (Fig. 6.57A). However, one limitation of this technique is the need for antigen to be negatively charged for electrophoretic movement within the agar matrix.
Counter current immuno-electrophoresis (CIE) relies on movement of antigen and antibody in opposite direction. The antigen carrying a negative charge migrates toward the opposite pole and the positively charged antibody toward the negative pole.
Antigen and homologus antibody are placed in separate cells in agar gel through which electric current is passed. Line of precipitin appears at a point where the two meet in 30 to 60 minutes (Fig. 6.57B). This test is faster and more sensitive than double diffusion technique. CIE is useful in detection of hepatitis B antigen and antibodies, DNA antibodies in SLE etc.
Agglutination Reaction:
Agglutination is an antigenic-antibody reaction in which an antibody interacts with particulate antigen to form a visible clumping (agglutinin). Antibodies that produce such reaction are called agglutinins. The reactions involved in agglutination are same as that happen during precipitation.
However, the formation of agglutinin takes much less time. Unlike the formation of precipitin particles like bacteria, erythrocytes, synthetic latex coated with antigen or antibody etc. can be used to detect either soluble antibodies or antigens, respectively. Better agglutination reaction takes place with IgM antibody than with IgG. Principles of lattice formation governing precipitation and agglutination are same.
Criteria for Agglutination:
i. Agglutination reaction depends on the valency of both antigen and antibody.
ii. Bilavent antibody like IgG inhibits precipitation reaction. For cross-linking, polyvalent antibody must be present.
iii. Excess of antibody may inhibit agglutination reaction which is known as pro-zone effect. At high antibody concentration, the number of antibody-binding sites may greatly exceed the number of epitopes present on antigen. As a result, antibodies can bind antigen only univalently instead of multi-valently. Antibodies that bind univalently cannot crosslink one antigen to another.
Mechanism of agglutination:
Mechanism of agglutination formation is same as that of precipitation. Multivalent antibody binds with bivalent or polyvalent antigen when both are present in optimal proportions.
Techniques of Agglutination:
1. Slide agglutination test:
This is an widely used method to detect particulate antigens like bacteria, red cells etc. A fine suspension of antigen is made in saline on a slide and a small drop of antiserum is added. The slide is rocked for a short time and clumps are formed within minutes.
This type of agglutination is of two types:
(a) Active agglutination test
(b) Passive agglutination test.
(a) Active agglutination test:
In this case, direct agglutination between antigen and its corresponding antibody occurs, e.g., agglutination of Salmonella typhi, Vibrio cholerae using specific antibody.
(b) Passive agglutination:
Agglutination test can be performed with soluble antigen also. In this technique, antigen coated red blood cells are prepared by mixing a soluble antigen with red blood cells that have been treated with tannic acid or chromium chloride, both of which promote adsorption of the antigen to the surface of the cells.
Sera containing serial dilution of antibody are placed in microtiter plate wells and the antigen-coated red blood cells are then added to each well. Agglutination is assessed by the size of the characteristic spread pattern of agglutinated red blood cells on the bottom of the well.
Inert, synthetic particles like latex beads; bantonite etc. may be used instead of RBC. Latex beads coupled with antigen may be used immediately or stored for later use. This test is useful for identification of bacteria and soluble extracellular bacterial antigens. Passive agglutination reactions are simple to perform, do not require expensive equipment and can detect small amount of antibody.
Agglutination Inhibition:
A very little quantity of antigen can be detected by a modified agglutination technique, called agglutination inhibition. In this case, absence of agglutination indicates the presence of antigen in a said sample. For example, urine or blood sample of an individual, suspected to have used some drug, is incubated with antibody specific for that drug. Then the red blood cells or other particles coated with the drug are added.
If the red blood cells are not agglutinated by the antibody, it indicates the sample contained an antigen recognised by the antibody, suggesting that the individual was using that drug. In this way, this technique can be used to determine if an individual is using certain types of illegal drugs like cocaine or heroin.
Similarly, early pregnancy can also be detected by using latex particles coated with human chorionic gonadotropin (hCG) and antibody to hCG; premarital test for determination of immune status with respect to rubella virus etc.