In this article we will discuss about:- 1. Meaning of Fatty Acids 2. Roles of Fatty Acids 3. Nomenclature.
Meaning of Fatty Acids:
Since fats and oils both contain glycerol, the differences between them must be due to differences in their fatty acid components. It is therefore customary to describe triacylglycerols in terms of their fatty acids. Fatty acids are long-chain, mono-carboxylic acids.
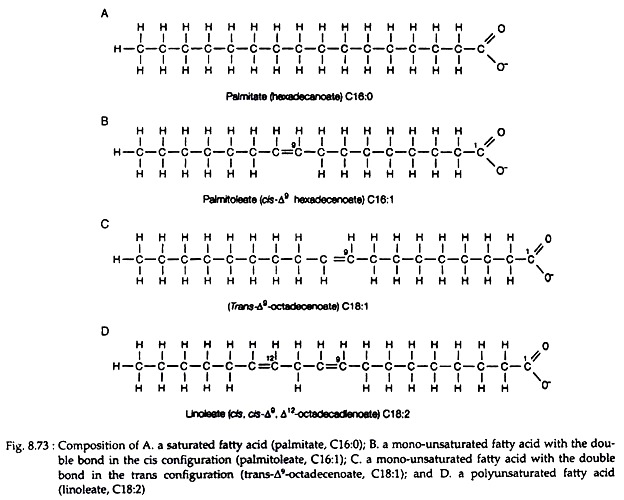
A fatty acid consists of a hydrocarbon chain and terminal carboxylic acid group (Fig. 8.73). Most of the fatty acids found in biological system have an even number of carbon atoms arranged in an un-branched, i.e., straight chain form. Chain length usually varies from 14 to 24 carbon atoms.
The fatty acids may be either saturated or unsaturated. A saturated fatty acid has all of the carbon atoms in its chain saturated with hydrogen atoms (Fig. 8.73A). This gives the general formula CH3(CH2)nCOOH, where n is an even number.
ADVERTISEMENTS:
The unsaturated fatty acids are mainly two types — mono-unsaturated fatty acids (MUFA) and poly-unsaturated fatty acids (PUFA). MUFA have one double bond in their structure (Fig. 8.73B and C), while PUFA have two or more double bonds (Fig. 8.73D). The double bonds in PUFA are separated by at least one methylene group.
The properties of fatty acids depend on their chain length and the number of double bonds. Shorter chain length fatty acids have lower melting temperatures than those with longer chains. Unsaturated fatty acids have lower melting temperatures than saturated fatty acids of same chain length.
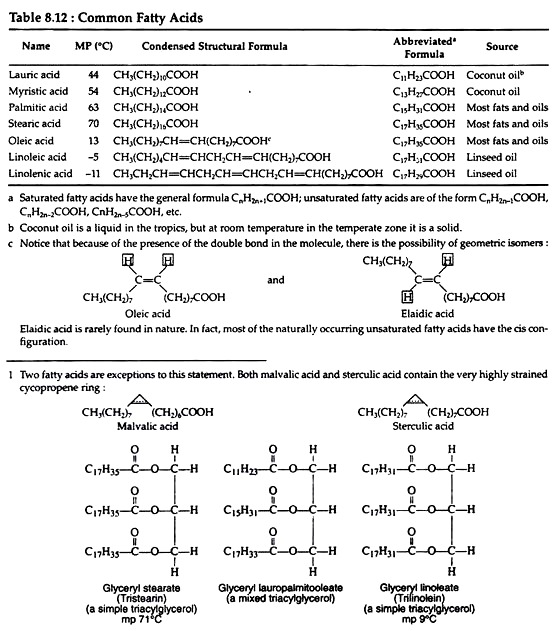
Notice that stearic acid, a saturated fatty acid, contains the same number of carbon atoms as the three unsaturated acids listed in the Table 8.12, yet it has a much higher melting point.
It is generally the case that the greater the degree of unsaturation of a fatty acid, the lower its melting point. This generalisation enables us to explain the differences between fats and oils. Fats contain a greater proportion of saturated fatty acids, whereas oils contain a greater percentage of unsaturated fatty acids.
Roles of Fatty Acids:
They perform four major biological functions:
1. They are participating component of glycerophospholipids and sphingolipids. These are essential components of biological membranes.
2. Numerous proteins are covalently modified by fatty acids.
ADVERTISEMENTS:
3. These are stored as triacylglycerols and act as stored fuel molecules. During necessity they are broken down to generate energy.
4. Derivatives of fatty acids serve as hormones (e.g., prostaglandins), and intracellular second messengers (e.g., DAG and IP3).
Nomenclature of Fatty Acids:
These are named according to the total number of carbon atoms, and to the number and position of any double bonds. The systematic names for fatty acids are made by adding — ‘-oic acid’ on to the name of the parent hydrocarbon.
A C-18 saturated fatty acid would be called octadecanoate, C-18 mono-unsaturated fatty acid would be called as octadecenoate and a C-18 fatty acid with two double bonds would be called as octadecadienoate (Fig. 8.73).
A fatty acid with 18 carbon atoms and no double bonds is written as C 18 : O, while one with 18 carbon atoms and two double bonds as C 18 : 2. The carbon atoms are numbered from the carboxylic acid end. So the position of double bonds can be described using the number of the first carbon involved in the bond, e.g., Δ9 shows a double bond between C-9 and C-10.
When two hydrogen atoms attached with the carbon atoms of the double bond are in the same side, they are called as cis (Fig. 8.73B & D). The most abundant fatty acids are given in Table 8.12. Fatty acids are usually called by their common names. They are derived from Greek or Latin words that indicate the source of the compound.
If all three hydroxyl groups of the glycerol molecule are esterified with the same fatty acid, the resulting ester is called a simple triacylglycerol. Although some simple triacylglycerols have been synthesized in the laboratory, they rarely occur in nature. All of the triacylglycerols obtained from naturally occurring fats and oils, contain two or three different fatty acid components and are thus termed mixed triacylglycerols.
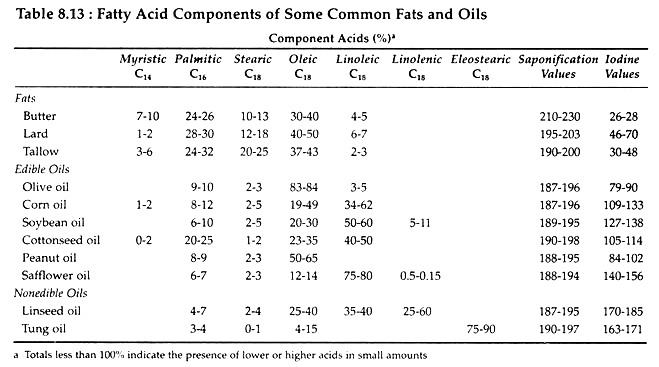
No single formula can be written to represent the naturally occurring fats and oils since they are highly complex mixtures of molecules. Table 8.13 shows the fatty acid composition of some common fats and oils. Notice that a fairly wide range of all values occurs. The range is wide because the composition of lipids is variable and depends upon the plant or animal species involved as well as upon dietetic and climatic factors.
For example, lard from corn-fed hogs is more highly saturated than lard from peanut-fed hogs. Linseed oil obtained from cold climates is more unsaturated than linseed oil from warm climates. Palmitic acid is the most abundant of the saturated fatty acids and oleic acid is the most abundant unsaturated fatty acid.