The below mentioned article provides notes on Urea Cycle.
Whenever amino acids are utilised for energy production or for the synthesis of glycogen or fat, an amino group is liberated in the form of ammonia. Living organisms must have some mechanisms of removing this ammonia from the cell environment because even low concentrations of ammonia are poisonous. (Levels of only 5 mg ammonia per 100 ml blood are toxic to humans.
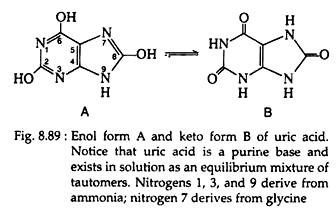
The normal concentration of this compound is about 1-3 µg per 100 ml) Organisms differ biochemically in the manner in which they excrete excess nitrogen. Most vertebrates excrete nitrogen as urea in the urine. Birds, reptiles, and insects convert nitrogen to uric acid (Fig. 8.89). All marine organisms, from unicellular to fish, excrete free ammonia.
Ninety-five percent of the nitrogenous waste products in humans results from amino acid catabolism; the remaining 5% comes from the metabolism of other nitrogen-containing compounds. It is interesting to note that the breakdown of purine bases in the human body results in the production of uric acid, and very small concentrations of this acid are found in the urine and in the body fluids.
ADVERTISEMENTS:
Under certain pathological conditions, large quantities of uric acid are produced and deposited as the sparingly soluble monosodium salt. Such deposits occur in the joints and produce the painful symptoms characteristic of gout.
The principal organ concerned with the formation of urea in mammals is the liver. Extensive liver damage results in the accumulation of ammonia and may lead to death. Once produced, urea is transported by the blood-stream to the kidneys and is eliminated in the urine. The normal individual excretes about 25 grams of urea daily, although this value varies greatly from day to day and is dependent upon protein intake.
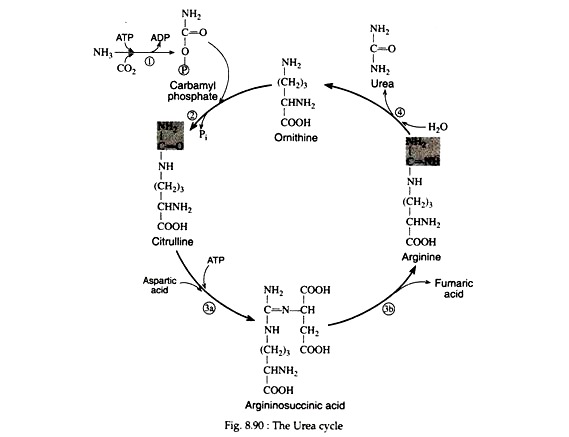
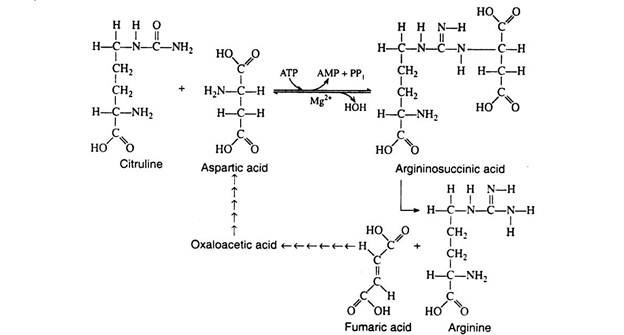
H. Krebs and K. Hensleit (1932) were the first to propose a Evelix sequence of reactions for the synthesis of urea. The essential features of this cycle, called the urea cycle, or the ornithine cycle, have remained unchanged and are depicted in Fig. 8.90.
Step 1:
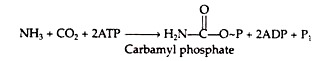
The formation of carbamyl phosphate from NH3 and CO2 is the initial step in urea synthesis. The reaction is catalysed by carbamyl phosphate synthetase. Energy for the process is supplied by two molecules of ATP. The reaction mechanism has not yet been elucidated.
Step 2:
ADVERTISEMENTS:
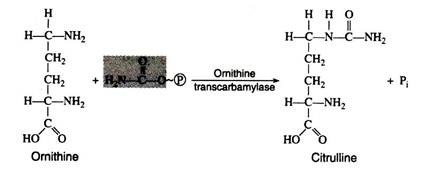
The high energy compound, carbamyl phosphate then reacts with ornithine in the presence of ornithine transcarbamylase to form citrulline. The high energy phosphate bond is cleaved in the process.
Step 3:
The conversion of citrulline to arginine involves two enzymatic seps. The first is the reversible condensation of citrulline with aspartic acid in the presence of argininosuccinic synthetase. The reaction produces argininosuccinic acid, a stable intermediate.
It is cleaved in a subsequent step to yield a molecule of arginine and a molecule of fumaric acid. The synthesis of fumaric acid is important since it can be converted via the Krebs cycle to oxaloacetic acid. The latter can be trans-aminated to yield aspartic and, or it can condense with acetyl CoA to form citric acid.
Step 4:
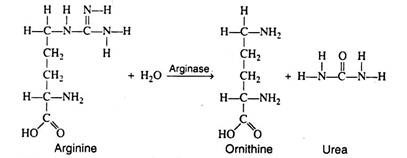
In the final step of the cycle, arginine is hydrolytically cleaved by arginase, yielding urea and regenerating ornithine.
The latter is now ready to accept another molecule of carbamyl phosphate, thus completing the cycle:
Notice that one of the nitrogen atoms that is eventually converted to urea is contributed by aspartic acid and not by ammonia.
However, NH3 may have been incorporated into the aspartic acid in the following manner:
The overall equation for the enzymatic synthesis of urea from ammonia, carbon dioxide, and the amino group of aspartic acid may be written as:
NH3 + CO2 + H2O + 3ATP + Aspartic acid → Urea + Fumaric acid + 2ADP + AMP + 2Pi + PPi