As carbon is the basic element of life, the carbon cycle is a very important biogeochemical cycle at the global level. This carbon cycle is characterised by a very active atmospheric reservoir pool that has been valuable to human interference, which, in turn, has changed the weather and climate in ways that has affected life on earth.
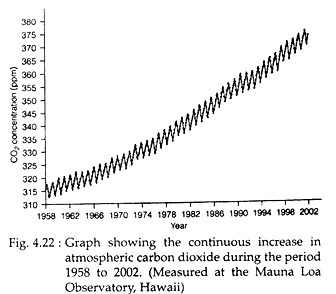
In the latter half of the twentieth century, the carbon dioxide concentration in the atmosphere has risen significantly (Fig. 4.22). This being an important greenhouse gas and with other such gases (methane, ozone, CFC etc.), has reflected the solar heat back down to earth—resulting in increase of temperature (global warming).
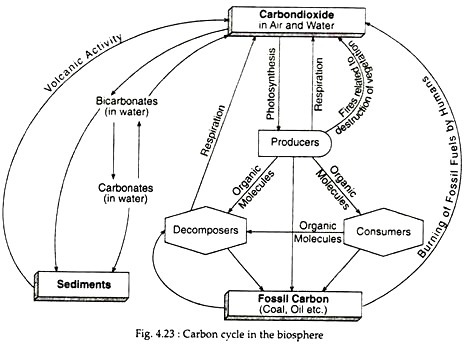
The basic part of the carbon cycle (Fig. 4.23) involves the taking up of carbon dioxide by plants from air and used in photosynthesis. Some carbon dioxide is returned back to the environment through respiration. Much of the carbon is retained in the plant body as organic compounds.
ADVERTISEMENTS:
Consumers, on the other hand, obtain their carbon when they eat the producers, while decomposers obtain their carbon when they act on the dead bodies of producers and consumers. The consumers and decomposers return carbon dioxide to air and water through their respiration.
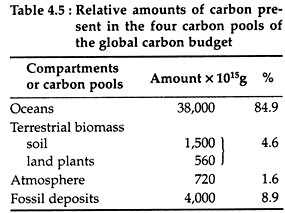
The above basic part represents only a small part of the carbon cycle. The modern carbon cycle comprises of four carbon pools—the oceans, the atmosphere, the terrestrial biomass and fossil deposits. The terrestrial biomass is further subdivided into the soil and land plant subunits. The carbon cycle depends not only on the relative amounts of carbon residing in each pool (Table 4.5), but also on the rates of flux among the pools.
ADVERTISEMENTS:
The four carbon sinks are:
(a) Ocean Pool:
ADVERTISEMENTS:
The oceans represent a carbon sink as they take in more carbon than they release. However, fresh water bodies may not be considered as sinks, as has been worked out by Jonathan Cole and co-workers (1994). They found that, globally, lakes are sources of carbon and not of sink. The oceans contain the largest carbon pool (Table 4.5).
The physical and biological processes within the vast water depths of the ocean controls the dynamics of the flux of carbon dioxide at the air-sea interface. Winds also ensures a rapid flux of carbon dioxide across the surface waters.
This carbon dioxide is used by phytoplankton in photosynthesis. The products of primary production thus formed moves from the surface to deeper waters, by a process referred to as biological pumping. This then lowers the carbon dioxide concentration at the surface water.
The oceans are heterogeneous with respect to circulation patterns, depth and temperature profiles. Carbon dioxide is roughly twice as soluble in water at 0°C than that at 20°C. This is due to the down-welling of cold, dense surface water which provides fresh entry of large quantities of carbon from the atmosphere.
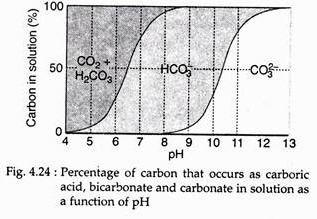
There is a complicated chemical system involving the oceans ability to dissolve carbon dioxide. It is in part related to the dynamics of biogenic carbonates in the water. When carbon dioxide dissolves, some of it combines with water to form carbonic acid.
This carbonic acid readily dissociates into ions of bicarbonates and carbonates:
The above occurs when the pH is high (Fig. 4.24). When the pH is low, abundant hydrogen ions in the sea water drive these reactions back, creating more carbonic acid. If calcium is present in the form of calcium carbonate in sea water, it dissociates into carbonate and bicarbonate ions.
The solubility of calcium carbonate is low under most conditions and is readily precipitated. However, it reacts with water and carbon dioxide to form the soluble bicarbonate.
All the above reaction are reversible; the overall effect is to buffer the carbon dioxide of the air. If carbon dioxide gets depleted in air, reversible reactions of the above equations take place resulting in the liberation of carbon dioxide from water to air.
Calcium carbonate is thus precipitated and their deposits are found only in shallow waters of the ocean. If carbon dioxide in air increases, the above reactions move to the right and more carbon dioxide dissolves in water. Materials thus become incorporated in sediments and returns to the system slowly. These may be brought back into circulation by geological processes such as volcanic activity (Fig. 4.23) or by the uplift of land.
(b) Atmosphere:
The atmosphere acts as a carbon sink. However, atmospheric carbon primarily in the form of carbon dioxide, carbon monoxide and methane, accounts for about 1.6% of global carbon (Table 4.5). The carbon- dioxide concentration has, however, shown an increasing trend (Fig. 4.22) as measured at Mauna Loa observatory in Hawaii from 1958 to 2002.
Before 1850, that is before the Industrial Revolution, the concentration of carbon dioxide in the atmosphere was 280 ppm. But during the last 150 years it has increased to more than 370 ppm. The burning of fossil fuel along with agriculture and deforestation has contributed to this enormous increase of carbon dioxide in the atmosphere.
Frequent plowing of agricultural soil has resulted in the release of carbon dioxide from the soil, which in large part is not compensated by the carbon dioxide fixed by crops. Thus, more carbon dioxide is added to the atmosphere than is removed. Forest removal may release carbon stored in woods. This is followed by carbon release from the oxidation of humus.
This increase of atmospheric carbon has led to concern regarding the greenhouse effect. This would increase the atmospheric temperature, resulting in global warming.
Carbon monoxide (CO) and methane (CH4) are the other two forms of carbon that are present in the atmosphere, in addition to carbon dioxide. Both carbon monoxide and methane arise from the incomplete decomposition of organic matter and can be oxidized to carbon dioxide. Carbon monoxide is not a global threat although it is deadly poisonous to human. The methane concentration has doubled over the past century mainly due to human activities. Methane is a greenhouse gas. It is 25 times heat absorbing as carbon dioxide and has the potential for increasing its contribution to global warming.
(c) Fossil Deposits:
Before the advent of modern man, the carbon cycle revolved around the exchanges between the oceans, the atmosphere and terrestrial biomes. The carbon locked in the fossil deposits was not involved in the cycle.
The storage of fossil carbon had resulted because the dead organic matters in bogs and certain other places had escaped decomposition. Although the amount of organic material thus formed seems to be small, its accumulations had taken place over a period of about 65 million years (ending 280 million years ago), leading to enormous accumulation of fossil carbon deposits. Such accumulation had taken place mainly during the Carboniferous period during which time most of our coal, gas and oil were stored.
With the discovery of such material being the source of energy, people resorted to its burning thereby resulting in the release of stored carbon. This resulted in the input of carbon into the global ecosystem. In 1900 the addition through burning of these fossil fuels was about 1 billion tons.
In 1955 it increased to 2 billion tons and in 1980 it was about 5 billion tons, or the release was at a rate of about 5 × 1015 g yr.−1. This was enough to raise the carbon dioxide content of the atmosphere by more than 2.5 parts per million each year.
The return of carbon to the fossil compartment occurs at a very slow rate which is much less than what is released into the global ecosystem. Thus, this excess carbon dioxide is tucked away in one of the three active carbon pools.
About 45% of the carbon released by the burning of fossil fuels (gasoline etc.), winds up each year into the atmospheric pool. Another 28% appears to enter the oceanic compartment. The rest has an unknown sink that absorbs about one billion tons of carbon each year.
Most probably the unknown sink of the global carbon cycle is the increasing terrestrial vegetation or biomass.
(d) Terrestrial Biomass:
The terrestrial biomass is subdivided into the soil and land plants. However, when accounting for the global carbon flux, they are often considered separately. Soil plays a significant role in the global carbon cycle. Soil also affects global nutrient cycling.
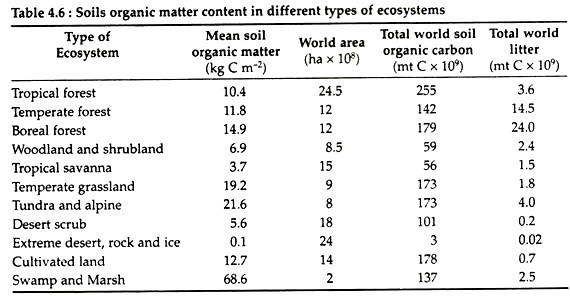
The primary source of organic matter in soils is dead plant material referred to as litter fall or leaf litter. The rate of accumulation of organic matter varies widely among various terrestrial ecosystems of the world (Table 4.6) and is dependent on the soil origin and the plant community in that area.
The total amount of organic carbon present in soils is about 1,456 × 109 metric tonnes. The decomposition of this organic material takes place most rapidly at the soil surface, resulting in the release of carbon dioxide into the atmosphere. Not all the organic material is decomposed.
Some become complexed with clay particles and become difficult for microbes to process. On the other hand, cultivation causes rapid decline of soil organic matter and some are lost in the runoff of water. A large portion of carbon dioxide is thus released into the atmosphere.
The land plant biomass serves as an additional carbon sink.
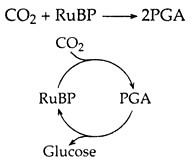
Certain plants with C3 photosynthesis, in mesic environments (with adequate water) assimilate the carbon in carbon dioxide into an organic molecule, in a single pathway referred to as Calvin-Benson cycle is given below:
It takes place in Mesophyll cell of plants. Here RuBP (ribulose bisphosphate) is a five-carbon organic compound and PGA (Phosphoglyceric Acid) is a three carbon compound. As the above step results in the formation of three-carbon compound, this process is said to be C3 photosynthesis.
The photosynthetic activity of these plants increases under conditions of elevated carbon dioxide concentrations in the atmosphere. Thus, it acts as carbon sinks. Even the terrestrial ecosystem of the arctic tundra, also can store large quantities of carbon and thus serve as carbon sink. It is also seen that young, rapidly growing forests are carbon sinks, so reforestation on a large scale might reduce the rate of global warming.
Global balance of carbon cycle:
In spite of the various carbon sinks, the concentration of carbon dioxide in the atmosphere has increased mainly due to the irresponsible attitude of human. The unchecked burning of fossil fuels and the destruction of terrestrial biomass has resulted in carbon inputs into the global ecosystem.
It is thus not clear whether the response of the global ecosystem to increased carbon release will be compensatory or will it result in a progressive destabilization of the ecosystem.