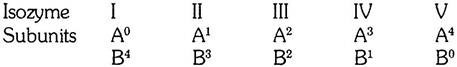In this article, the changing pattern of processes of differentiation will be shown in examples taken from the development of living organisms.
Hemoglobins:
How specific genes change their activity during development is illustrated by the composition of hemoglobins in man. The hemoglobin in adult humans is in the form of composite molecules consisting of four units (polypeptides), two of which are of the α type and two of the β type. Each type of polypeptide is produced by a separate gene.
The human fetus, however, has a different hemoglobin, which has been shown to consist of four polypeptides, two of which are identical with the α chains of adult hemoglobin; the other two have a different polypeptide sequence which has been designated as chain ϒ. Having a different polypeptide sequence, the ϒ chain is produced by a separate gene.
The amounts of fetal hemoglobin in blood diminish sharply after birth and are reduced to about 1 per cent for the rest of life. Instead, there appears the adult hemoglobin composed of the α and β chains.
ADVERTISEMENTS:
In view of the very direct relationship existing between the genes and the hemoglobin components, it is evident that after birth the gene producing the y chain is “switched off” and the gene producing the β chain is “switched on,” while the gene for the a chain continues to be active both before and after birth.
Lactate Dehydrogenases:
A somewhat similar situation to that of the hemoglobins is presented by the production, in mammals, of the enzyme lactate dehydrogenase. The enzyme has a molecular weight of 135,000, and the molecule can be split into four component polypeptides of about equal size. The isolated subunits do not have any enzymatic action.
The enzyme is commonly found in five forms, differing in electrophoretic behavior but having practically an identical enzymatic capacity. The five enzymes have therefore been called isozymes. Furthermore, it has been found that the subunits of the five isozymes are of two kinds only and that the subunits, named subunit A and subunit B combine at random to produce the five complete isozymes.
The composition of the isozymes can be represented in the following way:
The isozymes I and V are composed of four subunits of one kind only; the isozymes II, III, and IV contain both kinds of subunits in different proportions.
Unfortunately, the genetic background of the formation of the two subunits of lactate dehydrogenase is not yet known, but it is a reasonable assumption that each of the two kinds of subunits is coded by a separate gene. The subunits are then produced on ribosomes independently of each other and combine to form the five active isozymes.
If the combination of the subunits were random, and if both subunits were available in equal quantities, the five isozymes should be produced in the proportion 1:4:6:4:1. This has actually been confirmed experimentally by mixing together equal quantities of subunits A and B.
In the tissues, however, the proportions of the enzymes are not random, but depending on the particular tissue, the enzymes contain more either of component B or of component A. The first case is found in the skeletal muscles, while the kidney has a more balanced proportion of B and A. Furthermore, there is a general increase in isozymes with a high proportion of component B during life.
ADVERTISEMENTS:
It is thus seen that although both of the genes responsible for the production of dehydrogenase isozyme components are active practically in all cells of a mammal, their degree of activity changes from one tissue to another and in the course of an animal’s life.
Myosin:
The manufacture of specific substances may be correlated with visible changes in the organization of tissues in the embryo. This can be illustrated by considering the build-up of the protein myosin, which is the specific substance of muscle tissue. In the adult muscle, myosin amounts to from 10 to 12 per cent of the fresh weight or nearly 50 per cent of the dry weight.
The amount of myosin in the cytoplasm of the cells undergoing differentiation into striated muscle fibers has been estimated and compared with the morphological differentiation of the muscle.
In the rat embryo, the differentiation of striated muscle proceeds in the following way:
a. 12th to 13th Days after Fertilization:
The myotomes expand into the lateral and ventral body wall.
b. 14th Day:
The syncytial muscle fibers have been formed; on the same day the muscles become functional- they start contracting.
c. 17th Day:
ADVERTISEMENTS:
Transverse striation of the myofibrils becomes visible.
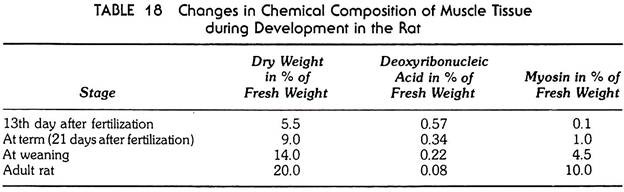
The changes in the chemical constitution of the muscle tissue during embryonic and postembryonic development are shown in Table 18.
Several conclusions can be drawn from the preceding data:
1. In the pre-functional stage, the amount of myosin in the presumptive muscle cells is very small, and this amount increases greatly in the period when the functioning of the muscle begins (between the thirteenth and twenty-first days after fertilization).
2. The differentiation of the muscle tissue is by no means accomplished when the tissue begins to function. This is shown by the tenfold increase in myosin in the postembryonic stages of development. This increase is due mainly to the elaboration of additional quantities of myofibrils in the muscle fiber. As a result, the strength of the fiber increases greatly. As measured by the breaking load of the fibers, their strength increases more than 400-fold between the seventeenth day after fertilization and the adult stage.
The specific physical organization of the myofibrils can be studied by testing the differentiating muscle for birefringence. Positive birefringence was found to be present in muscle tissue on the fourteenth day after fertilization. The birefringence is definite proof that the myosin molecules are arranged in long chains. This arrangement thus precedes the appearance of contractility, while the transverse striation of the fibrils appears after the muscles have started to contract.
The data on the development of striated muscle again stress the change in proportions between the nuclear apparatus and the functional mechanism of differentiating cells. While the amount of myosin (the functional substance) increases, the amount of the deoxyribonucleic acid decreases as compared with the other substances of the cells (fibers).
There is another lesson to be learned from the development of muscle tissue. It concerns the relationship between organ rudiment formation and histological differentiation. The organ rudiments—the myotomes, in this case—are formed in an early stage of embryonic development, about the seventh or eighth day after fertilization. The cellular materials for the development of the muscles take up their final position in the lateral and ventral body wall during the twelfth to thirteenth days of development. The elaboration of myosin and its arrangement in long chains (as shown by positive birefringence) follows only two days later.
Digestive Enzymes:
The development of enzymatic mechanisms may be illustrated by the appearance of the proteolytic enzymes, pepsin and trypsin, in the digestive tract of the salamander, Ambystoma punctatum. The gastric glands which secrete pepsin are developed in the walls of the stomach after all the parts of the alimentary canal are already clearly recognizable. In stage 40 the walls of the stomach consist of columnar epithelium, with a large number of yolk platelets in the cells.
At stage 41 the deeper-lying cells of the epithelium become clumped together, each clump representing the rudiment of one gastric gland. In stage 42 the cells of the gland rudiments are clearly arranged in a spherical layer, and a cavity appears in the middle—the lumen of the gland. In stage 43 the lumen of the gland is lined by a smooth cuboidal epithelium and communicates by means of a narrow duct with the cavity of the stomach. The yolk granules in the cells have disappeared by this time.
Pepsin first appears in the gastric glands between stages 42 and 43. Prior to and in stage 42 no trace of pepsin can be found in the stomach and at no stage either earlier or later is pepsin present in other parts of the body. We see that the glands are already clearly distinguishable morphologically (in stage 42) before they can perform their specific function, the production of pepsin. The morphological differentiation of the glands is, however, not quite complete by that stage, as there is some further progress till stage 43, when the glands attain their final structure.
The presence of trypsin in the pancreas can first be discovered in stage 43, slightly later than pepsin. The pancreatic acini first become distinguishable in parts of the organ (especially in the dorsal pancreas) at stage 41; they are more distinct in stage 42 and well differentiated in the dorsal pancreas in stage 43. As in the gastric glands, the structure of the secretory parts is laid down first, and the specific function (production of trypsin) sets in after that. It may be added that the mouth in salamander larvae breaks through at about stage 42, and feeding begins normally in stage 44.
Alkaline Phosphatase:
We shall now consider an enzyme which is more widely distributed in the body of the animal, namely, alkaline phosphatase, the enzyme which causes hydrolytic splitting of monoesters of the phosphoric acid in an alkaline medium and which may also function as a phosphotransferase; that is, it transfers the phosphate radical from one molecule to another. In early mammalian embryos, the enzyme is present in only small quantities and in a diffuse state, except that it seems to be always present in the nuclei of the cells.
At the time of the onset of differentiation, it appears in large quantities but in only a few tissues. It is found to be concentrated in the subcutaneous tissue of the embryo in cells concerned with the development of the subcutaneous connective tissue layer.
A little later, alkaline phosphatase is found in cartilages and in the hair papillae. In all three sites the enzyme is supposedly connected in some way with the elaboration of fibrous proteins—collagen fibers in the connective tissue and cartilage matrix and fibers of keratin in developing hairs.
In later stages of development, the enzyme is very abundant in the periosteum of bone and in the matrix of bone. In the latter position (where the enzyme is extracellular), the alkaline phosphatase splits off the phosphoric acid from glucose phosphates in the form of calcium phosphate, which impregnates the bone matrix. Other sites of alkaline phosphatase concentrations are in the brush border of proximal convoluted tubes of the kidney and in the cells of the intestinal mucosa.
In both of these sites, the alkaline phosphatase is concerned with the transfer of glucose from the lumen (of the renal tubule and the intestinal lumen respectively) into the internal medium of the body. In the adult, the quantities of alkaline phosphatase in the kidney, the intestinal mucosa, and the bone surpass by far the quantities found in other tissues.
It is worthwhile to trace the timing of the appearance of the alkaline phosphatase in the hair papillae. The rudiments of hairs in the mouse embryo first appear 14 days after fertilization in the form of epithelial thickenings. There is no trace of the connective tissue papilla in this stage.
In 15-day-old mouse embryos, mesenchymal cells accumulate under the epithelial thickenings, thus forming the rudiments of the future papillae. The differentiated papilla, contains large amounts of alkaline phosphatase, which is connected with the function of the papilla as the organ supplying the materials for the formation of the hair itself, which largely consists of fibrous keratin.
In the rudiment of the papilla, when it is first detectable, there is no alkaline phosphatase. Only in the more advanced hair rudiments of a 15-day-old embryo can small amounts of alkaline phosphatase be demonstrated. In the hair papillae of 16-day-old embryos, large quantities of the enzyme may already be found. The formation of the rudiment of the hair papilla thus precedes the appearance of the specific substance (enzyme) which is part of the functional mechanism of the differentiated organ.
Detection of New Products by Immunological Methods:
In cases in which the specific substances of differentiated tissues cannot be determined chemically, they can still be traced by the use of immunological methods. A suitable experimental animal, usually a guinea pig or rabbit, is immunized against the tissue that is being studied. For this purpose the tissue, crushed into a brie or in the form of an extract, is injected into the animal that is to be immunized.
The injected animal develops antibodies against the protein of the tested tissue in its blood plasma. The proteins which are used for immunization are called antigens. If the antibodies are again brought in contact with the same antigens, a reaction of a high degree of specificity will take place—the antibodies reacting only with the same antigen or with very closely related substances (and then to a weaker degree).
If a tissue extract containing antigens is mixed with the blood plasma of the immunized animal, a precipitation reaction takes place; the antigen is agglutinated by the antibodies and forms a precipitate.
The following experiment may serve as an example of the results obtained by the precipitation method. Experimental animals were immunized by injecting them with tissues of the adult chick. In this way anti-organ sera were prepared against brain, lens, kidney, bone marrow, erythrocytes, ovaries, and testes.
The antisera were then tested against tissue extracts from organs of the chick embryo at different stages of development. A reaction between the two proves that the specific tissue substances (antigens) of the adult animal are already present in the organs of the embryo.
The following list shows the earliest stage (expressed in days of incubation) at which the specific adult tissue antigens could be discovered:
In every case the antigen was found to be present when the tissues were well on their way to histological differentiation. Some “cross reactions” were also found, that is, reactions of an antiserum with tissue other than the one used for immunizing the donor of the serum.
The techniques used in the early experiments were very crude, but since then they have been improved in various ways. On the one hand, the detection of the antibody-antigen reaction has been made much more sensitive. The reaction of precipitation is observed while the two substances (the antigen and the antibody) are diffusing against each other in a gel. Alternatively, the antibody is conjugated with a fluorescent substance, and applied to sections of the tissue that is being tested.
The binding of the antibody to molecules of the antigen in the tissue can then be observed under the microscope if the tissue section is illuminated by ultraviolet light. On the other hand, the substances in the tissues can now be purified by various techniques, such as electrophoresis, and these purified substances, instead of crude homogenates of tissues, are used to elicit the formation of antibodies in the immunized animals.
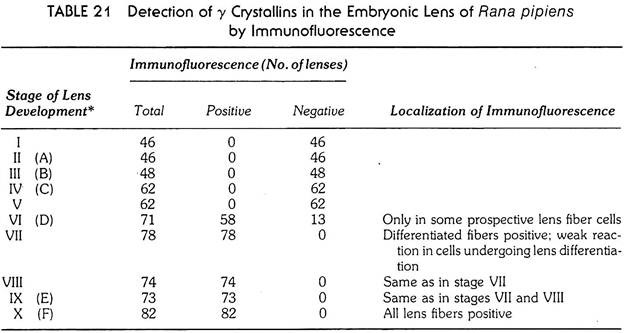
The improved techniques have been applied to the study of the appearance of specific proteins in the rudiment of the lens in amphibian embryos. The lens fibers, so characteristic for the structure of the lens contain three or four groups of proteins. In amphibians these are the α, β and ϒ crystallins, of which the ϒ crystallins (or ϒ crystallin, as this is almost a pure substance, with a molecular weight of 20,000) are predominant during the earlier stages of development. Antibodies were prepared either against total lens protein, or against purified ϒ crystallin, and tested on lens rudiments of successive stages of development.
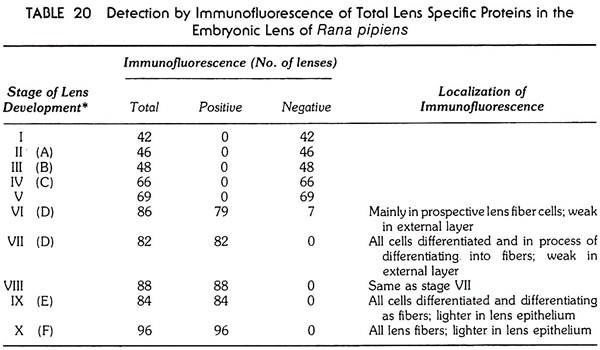
In both a newt, Triturus and the frog, Rana pipiens, the lens crystallins appear first at the stage when the cells in the proximal part of the lens rudiment start elongating—the first sign that the morphologically discernible lens fibers are starting to differentiate (see Tables 20 and 21). The stage in which the lens crystallins are first detected (stage VI in Tables 20 and 21).
It is to be noted that the proteins specific for the organ (the lens crystallins in this case) are produced in the tissue after the rudiment of the organ has already been formed by morphogenetic movements and are an expression of the functional differentiation of the organ.