In this article we will discuss about the gastrulation and formation of primary organs rudiments in birds.
The earlier stages of the discoidal cleavage in the eggs of reptiles and birds resemble essentially the cleavage in meroblastic eggs of the fishes. The blastoderm is several cell layers thick, and lies over a “sub-germinal cavity.” At a later stage of development an essential change becomes apparen – the blastodisc becomes subdivided into a central part, called the area pellucida, and a surrounding zone, called the area opaca.
The terms “pellucida” and “opaca” refer to the appearance of the two areas in the living embryo – the central area appears transparent (pellucid), while the peripheral zone appears opaque. The differentiation of the blastodisc into the area pellucida and area opaca has been best investigated in the embryos of the hen.
The transformation occurs while the egg is still in the uterus of the laying hen. About 12-14 hours after the egg reaches the uterus (and thus 6-8 hours before the egg is laid), some cells on the inner (under) side of the blastoderm become detached from the rest of the blastoderm and fall into the sub-germinal cavity.
ADVERTISEMENTS:
The cells to do so are the ones which contain the greater amounts of yolk; the cells remaining in the intact blastoderm are the ones with less yolk. This shedding of the yolky cells from the blastoderm begins at what will be the posterior edge of the area pellucida and gradually spreads forward, until the whole of the area pellucida becomes free of yolky cells.
As a result, the epithelial layer in the region of the area pellucida becomes thinner, consists of fewer layers of cells, and—the cells containing little yolk—the epithelium becomes transparent. In the area opaca the yolky cells are not shed, remain in the epithelial layer, and render this part of the blastodisc opaque.
Incidentally, the order in which the area pellucida becomes devoid of yolky cells indicates the orientation within the blastodisc of the future embryo – the point where the yolky cells start to be shed will be the posterior end. Thus, the bilateral symmetry of the embryo becomes established.
The yolky cells in the sub-germinal cavity appear to be resorbed later. Only the area pellucida furnishes material for the formation of the body of the embryo. The cells of the area opaca are concerned with the breakdown of the underlying yolk and thus indirectly supply the embryo with foodstuffs.
ADVERTISEMENTS:
Gastrulation and the formation of the germinal layers in the birds is a very complicated process that proceeds in several steps. After the completion of cleavage and the subdivision of the blastoderm into an area pellucida and area opaca, a second thin layer of cells appears beneath the original epithelium of the area pellucida.
A similar development is observed in most reptiles, especially lizards and snakes, but not in tortoises. In the hen this second layer starts forming just before the egg is laid, and is completed in the first hours after laying. The presence of this lower layer of cells has caused much controversy among embryologists as to its origin, significance, and homologies.
The two layers certainly do not represent ectoderm and endoderm respectively. It has been found advisable, therefore, to refer to the two layers by the terms epiblast (for the upper layer of cells) and hypoblast (for the lower layer of cells). In fact, the upper layer gives rise to all three germinal layers of the embryo (the ectoderm, mesoderm, and endoderm).
It has therefore been concluded that the two-layered embryo of birds and reptiles is still in the blastula stage and that the lower layer, the hypoblast, corresponds to the cells lying on the floor of the blastocoele cavity in animals such as amphibians.
ADVERTISEMENTS:
The cavity between the epiblast and the hypoblast of birds and reptiles thus corresponds to the blastocoele of amphibians and fishes. The space between the hypoblast and the yolk may then be distinguished as the sub-germinal cavity.
The origin of the cells constituting the hypoblast is very difficult to ascertain by experimental methods, since the eggs, being fertilized internally, start cleaving and may reach the blastula stage while they are still in the oviducts.
This difficulty has been overcome, however, as it has been found that the egg can be pressed out of the hen’s uterus manually, and its development observed, either while allowing the egg to develop at a temperature of 42° C. (the body temperature of the hen), or after excision of the blastoderm and its cultivation in vitro.
It is a recognized fact that the hypoblast first appears in the posterior part of the area pellucida and extends forward in later stages. The most plausible interpretation of hypoblast development is that it originates from loosely arranged cells lying internally at the border between the area pellucida and area opaca.
The cells are at first lying free under the epiblast, but gradually they link up with each other, forming a continuous layer of flattened cells, starting from the posterior end. In this way the layer spreads in an anterior direction. By applying carbon marks to the developing hypoblast it was found that in the process of its formation the cells of the hypoblast shift in a forward direction, thus contributing to the completion of the hypoblast layer under the anterior half of the area pellucida.
Perhaps the formation of the hypoblast may be likened to the “pre-gastrulation movements” in the amphibian egg, inasmuch as they precede the processes of gastrulation proper; that is, the movements which result in the formation of the three germinal layers, the ectoderm, mesoderm, and endoderm.
Numerous experiments have been conducted to study the fate map and the gastrulation movements in the bird embryo. The method of local vital staining, as applied with success in the study of amphibian gastrulation, was used in the earlier work on bird embryos.
The results have been disputed, since vital stains tend to diffuse and thus could give misleading results. An alternative method has been to mark portions of the blastoderm with fine carmine or carbon (charcoal) particles which stick to the surface of the cells and are carried around by the cells during their movements.
The particles, however, do not become constituents of the cells and could possibly be shed by the cells on their way, at least in some cases. The uncertainties in experimental results have been augmented by the fact that the carbon markings were made on blastoderms cultivated in vitro, removed from the egg and placed, usually upside down, on the surface of agar gel or coagulated albumen.
ADVERTISEMENTS:
The expansion of the blastoderm, as in normal development, does not proceed on agar blocks. The most reliable method was found to be to mark parts of the blastoderm with radioactive tracers (tritiated thymidine). The method is somewhat complicated.
First, an explanted blastoderm is immersed in a medium containing tritiated thymidine. In three to eight hours the tritiated thymidine is incorporated into the chromosomal DNA of the growing and reproducing cells of the embryo. The embryo labeled with tritiated thymidine now serves as a donor.
To establish the fate and the movements of a particular part of the embryo, a recipient embryo is chosen which is in the same stage of development as that attained in the meantime by the donor. A small area of the recipient embryo is then excised and replaced by a corresponding piece from the donor embryo.
Healing usually occurs quickly, and the development is not disturbed. The chromosomal DNA does not pass out of the nuclei of the labeled cells but remains in the chromosomes of their descendants, though it is gradually diluted with each subsequent replication of the chromosomes.
In spite of this the radioactivity remains for a considerable time, and if the embryos are later fixed and sectioned, autoradiograms can be prepared by coating the sections with photographic emulsion. Silver grains in the developed emulsion provide unequivocal evidence that particular cells or groups of cells are descendants of the original graft.
Unfortunately, the work has to be done on embryos cultivated in vitro. In addition, having to label the donor embryos for several hours before the operation precludes the study of blastoderm development immediately after the eggs are laid, but only after some further development had taken place.
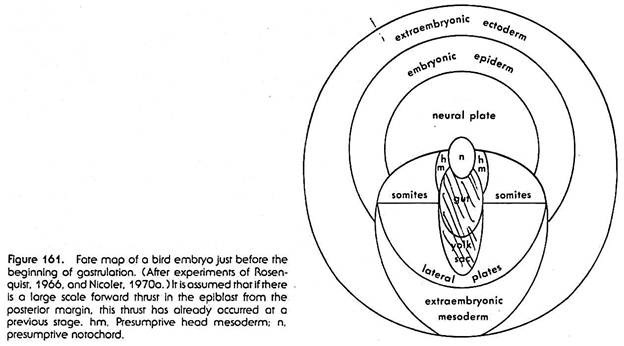
Nevertheless, by the use of tritiated thymidine marking, fate maps of bird embryos have been obtained which are far superior in detail and reliability to any which have been based on vital staining or carbon particle marking. The fate map in Figure 161 is based on this work. The map refers to the epiblast only.
Roughly in the center of the area pellucida lies a small area which will produce the notochord. Posterior to this, in the median plane of the embryo, lies an elongated oval area which is the presumptive endoderm. The part nearer to the notochordal area is incorporated in the body of the embryo proper, and it produces the gut with its subordinate parts.
Further toward the posterior edge of the area pellucida lies the extra-embryonic endoderm which will form part of the lining of the yolk sac. To the right and left of the presumptive notochord and endoderm and also posterior to the presumptive extra-embryonic endoderm lie the various subdivisions of the presumptive mesoderm.
Nearer to the notochordal area is situated a not very precisely defined section producing the prechordal (head) mesoderm. Posterior and lateral to that section are the presumptive somite areas, and posterior to these is the presumptive lateral plate mesoderm; the right and left lateral mesoderm areas are in continuity with each other posterior to the endodermal area.
Toward the posterior edge of the area pellucida a fairly large crescentic area is situated which will give rise to extra-embryonic mesoderm, particularly to the extra-embryonic blood vessel system. The presumptive ectoderm occupies the anterior and lateral parts of the area pellucida. A roughly semicircular area anterior to the notochordal material is the presumptive neural system.
In a band surrounding this is the presumptive embryonic epidermis (including material for the various epidermal derivatives). Still further outward, forming almost a complete ring at the outer edge of the area pellucida, lies the extraembryonic ectoderm—ectoderm which will not take part in building the body of the embryo proper. This it has in common with the entire area opaca.
It will be convenient at this stage to refer to the origin of the bilateral symmetry in a hen’s egg. The father of embryology, Karl Baer, had made the observation that if the hen’s egg is viewed with the pointed end to the right of the observer, the embryo in the egg will be found to lie transversely to the long axis of the egg, with the head pointing away from the observer.
This orientation of the embryo is the result of the rotation of the egg within the uterus of the hen, where the egg stays for about 20 hours before being laid. In the uterus the egg is slowly rotated around its long axis, 10 to 15 revolutions per hour. The distribution of mass in the yolk does not allow it to rotate with the rest of the egg (egg white, membranes and shell); the yolk remains stationary, with the blastodisc on top, but not quite in a horizontal position.
Due to friction of the rotating egg white, the yolk is turned slightly, so that the blastodisc is inclined in the direction of the rotation. It has been established that when the embryo starts developing within the blastodisc, its posterior end is formed at the edge of the blastodisc which is held uppermost during the period of rotation.
This is first noticeable when the area pellucida is starting to form by the thinning out of the original blastoderm, and then when the hypoblast and subsequently the primitive streak begin to develop at the same end. The rotation in the uterus is counterclockwise as viewed in the direction toward the cloaca.
If the egg is oriented with the pointed end toward the cloaca, as is the general rule, the direction of rotation and the resulting slanting position of the blastodisc account for “Baer’s rule” for the orientation of the embryonic axis. In exceptional cases the egg is oriented with the blunt end toward the cloaca and then, due to the same rotation, the embryo develops in the opposite position to that demanded by “Baer’s rule.”
It is not the rotation in itself that determines the orientation of the embryo in the egg, but the oblique position of the blastodisc during the crucial stages of development. This has been proved by extracting the egg from the uterus, and placing it in a vertical position.
The chalazae prevent the egg yolk from turning so that its animal pole comes to lie on top, with the result that the blastodisc assumes a stable slanting orientation. Due to this orientation the embryo develops with the posterior end at the uppermost edge of the blastodisc and with its main axis parallel to the long axis of the egg.
By placing eggs in different positions at various intervals after their entry into the uterus, the exact time has been found when the polarity of the blastodisc becomes determined. Eggs extracted after 10 hours in the uterus were placed first in a vertical position with their pointed ends up, and after varying periods of time the position was reversed; that is, the eggs were placed with their blunt ends up.
If the reversal was made after four to five hours (total uterine age 14-15 hours), the embryos developed according to their latter position, that is with the rear end toward the blunt end of the egg. If the reversal was made after eight hours in the first position, the embryo developed with its rear end toward the pointed end of the egg. Thus, the second position (blunt end up) was no longer capable of changing the orientation of the embryo. The orientation was already finally determined.
With the changeover in the period of 5½ to 7 hours, the axis of the embryo took up an intermediate and variable orientation, this time 15½ to 17 hours of uterine life of the egg—is the period when the main axis of the embryo becomes gradually determined, depending on the orientation of the blastodisc in space; that is, in relation to the force of gravity. At the time when this determination is taking place the area pellucida is just being formed.
The presumptive areas of the blastoderm take up their positions in the embryo by means of a series of morphogenetic movements. One of these, is the forward movement in the hypoblast.
Even after the hypoblast is complete, the forward streaming continues; from the region at the posterior end of the area pellucida the hypoblast cells flow in a “fountain-like” fashion spreading out toward the anterior and lateral edges of the area pellucida. Some of the hypoblast cells are embedded in the thickened border between the area opaca and the area pellucida at the anterior edge of the latter.
Several hours after the beginning of incubation of the laid egg, a thickening can be noticed at what will be the posterior edge of the area pellucida. This is the same place at which the thinning out of the area pellucida started during the intrauterine development of the egg.
It marks the beginning of the processes that lead to gastrulation and the formation of the three germinal layers in the bird embryo. Since the organ-forming part of the blastoderm in birds occupies only a limited area inside the blastodisc (only the central part of the area pellucida), the invagination of the endoderm and mesoderm takes place in a special region inside the area pellucida.
This region is a median strip of blastoderm, starting from the thickening, at the posterior edge of the area pellucida and eventually extending to about three fifths or three quarters of the entire length of the area. This strip of blastoderm becomes thickened during the stages of gastrulation and is known as the primitive streak.
Along the middle of the primitive streak, when it is fully developed runs a narrow furrow, the primitive groove. At the anterior end of the primitive streak there is a thickening, the primitive knot or Hensen’s node. The center of Hensen’s node is excavated to form a funnel-shaped depression. In some birds (the duck, for instance) this depression extends forward into a narrow canal.
The thickening of the blastoderm is brought about by a convergence of its surface layer, the epiblast, toward the midline in the posterior half of the area pellucida.
As the cells nearest the midline become concentrated to form the early primitive streak, the parts of the epiblast lying farther out laterally and anterolaterally swing in a curve backward and inward to take the place of parts of the blastoderm shifting toward the midline. The primitive streak first becomes visible in the hindmost part of the area pellucida and is designated the short primitive streak.
The primitive streak now elongates by the concentration of more and more material from the sides toward the midline in front of the original short primitive streak. The early primitive streak is broad and its edges are somewhat vaguely indicated.
In later stages it contracts in a transverse direction, becoming narrower and quite sharply delimited, and is called the definitive primitive streak. In the process of this transformation, the primitive streak elongates and its anterior end is pushed even farther forward, though the amount of this forward thrust has been a matter of controversy among embryologists.
The movements in the blastoderm leading to the final placement of cells in the hypoblast and to the formation of the primitive streak in the epiblast may be called pre-gastrulation movements, to distinguish them from the gastrulation movements proper.
At the stage of the short primitive streak, the cells of the blastoderm already begin to migrate into the space between the epiblast and the hypoblast. The process of translocation is different from that in Amphioxus and in the amphibians. There is no in-folding of the epithelial layer of the blastoderm.
In the birds, the cells seem to be moving downward singly, even if there are many cells moving in the same direction. The regular epithelial arrangement of cells found in the epiblast is lost. This type of gastrulation movement bears the name of immigration.
Soon the migrating cells reach the hypoblast and establish intimate contact with the cells of this layer. Henceforth the entire primitive streak is a mass of moving cells. The direction of movement is mainly downward from the surface of the blastoderm toward the hypoblast, but the mass of migrating cells also spreads out sideways and forward from the anterior end of the primitive streak.
Although the cells of the chick blastoderm move individually, their movements are obviously directed by common causes, and therefore the whole mass of cells moves in a coordinated fashion. The formation of depressions on the surface of the blastoderm, the furrow along the midline of the primitive streak, and the funnel-shaped depression in the center of Hensen’s node are due to this mass movement of cells from the surface of the blastoderm into the interior.
As the cells of the epiblast migrate into the interior, whole areas of the blastoderm disappear from the surface. They are replaced, however, by the adjoining areas moving toward the midline and taking their places in the primitive streak. The newly arrived cells in their turn migrate down into the interior.
Thus the primitive streak persists, although the cells of which it is made do not stay in the same place but are constantly replaced. The first areas to start ingression into the interior are the presumptive endoderm, the notochord, and the presumptive head mesoderm.
The presumptive notochordal cells become concentrated in the definitive primitive streak in the deeper parts of Hensen’s node. They then start moving as a dense mass in the midline straight forward from Hensen’s node underneath the surface of the epiblast.
The mass of notochordal cells can be distinguished from the surrounding (mainly mesodermal) cells and is called the head process, or more correctly, the notochordal process. The narrow canal mentioned previously which is found in some birds (e.g., the duck) penetrates into this notochordal process. Since formation of the canal is due to the movement of the invaginating cells of the gastrula, the cavity of the canal must be recognized as corresponding to a part of the archenteron, even though the lining of the canal consists exclusively of presumptive notochordal cells.
There is no other cavity in the development of birds that could be considered as a homologue of the archenteron. The invagination of the mesoderm or the endoderm does not lead to the appearance of a cavity. In other vertebrates the archenteron may also be completely absent.
The endoderm starts immigrating at an early stage, even before the primitive streak is fully formed. Cells of the presumptive endoderm penetrate into the hypoblast and push outward and forward the cells originally composing this layer. As a result, an extensive part of the hypoblast around and in front of the Hensen’s node becomes replaced by cells derived from the primitive streak.
This area shifts forward and later gives rise to the foregut. The endoderm lying in the more posterior part of the primitive streak after invagination moves farther laterally, and even in a laterocaudal direction, replacing the original hypoblast and later giving rise to part of the lining of the yolk sac.
As the notochordal and endodermal materials disappear from the surface, the presumptive somite areas converge medially and enter the primitive streak at its anterior end, behind Hensen’s node. From this part of the streak somite material invaginates into the interior.
After passing into the interior, the cells of the presumptive somites migrate outward and forward and become distributed in strips on each side of the notochordal process. The presumptive lateral plate mesoderm likewise enters the primitive streak after disappearance of the endodermal area from the surface.
The part of the streak occupied by lateral plate mesoderm is immediately posterior to the position in the streak of the somite mesoderm and forms the middle section of the primitive streak throughout the greater part of the period of gastrulation. Having migrated inward the material of the lateral plate’s shifts laterally and forward, arranging itself to each side of the somites. The material for the heart and kidneys moves in together with the lateral plate mesoderm.
The posterior part of the primitive streak (about two fifths of its length, when the streak is fully developed) contains only material for extra-embryonic mesoderm.
After its first appearance, the primitive streak increases somewhat in length, owing mainly to an elongation of its posterior half. The adjoining part of the area pellucida is also involved in the stretching, so that the area pellucida loses its circular shape and becomes more or less pear-shaped, the attenuated end being directed posteriorly.
The elongation of the primitive streak is, however, only temporary – As the cells destined to become notochord, endoderm, and mesoderm migrate into the interior of the embryo, the material of which the primitive streak consists becomes gradually exhausted. The influx of cells from the sides becomes retarded and can no longer compensate for the expenditure of cells due to immigration.
The whole primitive streak begins to shrink, the anterior end receding backward, while the posterior end remains more or less stationary. It has been ascertained by marking with carbon particles and by tritiated thymidine marking that Hensen’s node is carried backward bodily during this recession of the primitive streak.
The presumptive notochordal cells contained in Hensen’s node one after another continue migrating downward and forward, so that the notochordal process is prolonged backward, owing to continual apposition of new cells. A part of the presumptive endodermal area remains just posterior to Hensen’s node.
As the node retreats, cells from this area continue moving inward and form a strip of definitive endoderm underneath the notochord. This endodermal strip gives rise to the middle and posterior sections of the embryonic gut. In the same way, the strip of somite mesoderm and the sheets of lateral plate mesoderm extend backward from parts of the primitive streak which retain their properties although the whole structure is reduced in scale.
By the end of gastrulation, the primitive streak has shrunk almost to nothing; the residue becomes partly incorporated into the tail-bud, which is formed at the posterior end of the body of the embryo, and partly into the cloacal region of the embryo.
As the primitive streak recedes backward, the neural system area, which lies just in front of Hensen’s node and even forms the most anterior half of the node itself, stretches backward, while the lateral horns of the area are drawn in toward the midline. As a result, a strip of presumptive neural plate is left in the wake of the receding primitive streak. A part of the neural plate area is also found in front of the place where Hensen’s node was located originally. This part will become the prechordal part of the brain.
The primitive streak, being the pathway by which the presumptive internal organs are invaginated into the interior of the embryo, must be considered as the blastopore, even if it does not lead into an archenteron. The recession and disappearance of the primitive streak correspond therefore to the closure of the blastopore. The remnant of the blastopore, as in the amphibians, is to be found in association with the anus (or cloaca).
We have seen that the primitive streak, while it is active, is a mass of cells continuous with both the epiblast and the hypoblast, as well as with the sheets of mesodermal cells migrating in between the two into the blastocoele. Cells from right and left, and also to a certain extent from above and below, intermingle within the primitive streak.
There is unequivocal evidence that cells situated originally in areas on one side of the streak, after passing through the streak, may come to lie on the opposite side of the embryo. It has also been found that some cells from the hypoblast find their way into the primitive streak and Hensen’s node, and eventually into the notochordal rudiment.
In the part of the embryo from which the primitive streak has receded, the continuity of all three layers no longer occurs. The sheet of mesodermal cells, together with the notochordal process, is separated from the overlying epithelium, which no longer contains presumptive mesoderm or endoderm and from then on is pure ectoderm. Likewise, the chordomesodermal mantle has been split off from the endodermal layer underneath.
The hypoblast of the early embryo, without losing its continuity, has been completely transformed by the immigration of endodermal cells from the epiblast. A median strip has been replaced by cells from the epiblast, and these are the only cells from which the gut and its derivatives are formed. The original hypoblast cells contribute to the development of extra-embryonic parts, such as the yolk sac, and even the latter is lined in part by cells migrating from the primitive streak.
The fact that the gut of the embryo is derived from the epiblast, and not the hypoblast, justifies our claim previously made that the epiblast cannot be equated with ectoderm, or the hypoblast with endoderm.
Neurulation and the formation of the other primary organ rudiments proceed in an anteroposterior direction, just as in the fishes. The neural plate appears in the brain region while the gastrulation movements are still in full swing. As Hensen’s node recedes, farther and farther parts of the neural plate become differentiated, and the anterior parts of the neural plate proceed to close into a tube, the neural tube.
The neural tube is formed in a typical way, with a large cavity enclosed by the walls of the tube. The notochord becomes separated from the adjoining sheets of mesoderm, and the dorsal mesoderm becomes subdivided into segments—the somites.
The presumptive alimentary canal of the embryo is represented by a narrow median strip of the endodermal layer. Folds directed downward and inward arise on both sides of this strip; the folds approximate each other and fuse, enclosing a cavity which will be the lumen of the alimentary canal.
This process, however, does not take place along the whole length of the embryo but only at its anterior and posterior ends. In the middle part, although a groove in the endodermal epithelium may be present, connecting the anterior and posterior portions of the alimentary canal, this groove continues to be open toward the underlying yolk. The fate of this opening will be considered later in another connection.
The sheet of mesoderm invaginated through the primitive streak at first consists of loosely lying cells. At the stage when the segmentation of the mesoderm begins, or shortly before, the mesodermal cells reunite into an epithelial arrangement. After the somites and the lateral plates have been formed, the mesoderm of the lateral plate splits into two layers – the external or parietal layer and the internal or visceral layer.
The cavity between the two layers is the coelom. Small coelomic cavities also appear in the somites, but these cavities, as in the amphibians, soon disappear again. The segmentation of the dorsal mesoderm to form the somites starts at the anterior end of the body and proceeds gradually in a posterior direction.