In this article we will discuss about:- 1. Introduction to Metamorphosis in Insects 2. Causation of Molting in Insects 3. Factors Controlling 4. Mechanism of Action of Insect Hormones.
Introduction to Metamorphosis in Insects:
In any consideration of metamorphosis in insects, it must be taken into account that metamorphosis in these animals is a special form of molting (the periodic shedding of the cuticle of the skin which necessarily accompanies growth, because the strongly sclerotized parts of the cuticle cannot stretch or cannot stretch sufficiently to accommodate the growing mass of the body).
A large proportion of the external features of an insect is embodied in the sclerotized parts of the cuticle, such as the details of shape of parts of the body, the hairs and spines on the surface of the skin, the sculpture of the surface of the cuticle, and to a certain extent, the pigmentation. In the process of molting, these features are lost with the discarded cuticle.
The external characters of the insect, as far as they find their expression in cuticular structures have to be produced anew at each molt, though on a larger scale. The new cuticle is secreted by the epidermis of the skin, and therefore it is this layer of cells which is directly responsible for the external features of the insect emerging from a molt, whether the molted insect is an enlarged copy of the previous stage or whether it shows some new characters.
ADVERTISEMENTS:
The molt in every case is quite a complicated process. In between two molts the cells of the epidermis are quiescent, they are more or less flat, and the epithelial layer may be rather thin. The epidermal cells adhere closely to the inner surface of the cuticle. Before each molt, however, they become activated, detach themselves from the cuticle, and enter a phase of rapid growth and proliferation.
Numerous mitoses are observed. (Proliferation of epidermal cells is not observed, however, during the larval molt of cyclorraphe dipteran larvae, in which the cells of the larval epidermis do not divide between the stages of the egg and the pupa and are eventually discarded and replaced by imaginal epidermis.)
The number of epidermal cells produced by mitosis may be in excess of what is necessary, and some of the cells at this stage undergo degeneration by pyknosis. In spite of the degeneration of some of the cells, the layer of epidermis becomes thicker, and the remaining cells become arranged in a regular columnar epithelium.
The surface of this epithelium foreshadows the shape of the animal emerging from the molt. In those parts of the body which are to be increased as a result of the molt, the epidermis is thrown into folds which expand and straighten out after the insect has emerged from its old skin. The folding is especially great where new or greatly increased appendages (such as the wings) are to be developed.
ADVERTISEMENTS:
The epidermal cells then produce on their surface a thin layer of hardening secretion which becomes the outermost layer of the new cuticle, the epicuticle, consisting of a substance of lipoprotein nature, cuticulin. A fluid produced mainly by special molting glands is now poured into the space between the surface of the new epicuticle and the innermost surface of the old cuticle. The fluid contains enzymes which digest the inner layers of the old cuticle until little more than the old epicuticle is left.
The fluid with the substances digested from the old cuticle later becomes reabsorbed into the body of the insect. At the same time that the old cuticle is being digested, the epidermis produces further layers underneath the new epicuticle – the exocuticle, containing large quantities of cuticulin and also phenolic substances which are later oxidized to produce the dark pigment in the cuticle, and eventually the endocuticle, consisting of protein and chitin, which is a nitrogenous polysaccharide.
When the old cuticle is reduced to a thin shell, it is ruptured at the back of the head and thorax, and the insect crawls out of its old coat. The new cuticle is by no means complete at this stage- after molting the cuticle hardens, and visible pigment is produced in it from colorless precursors (phenolic substances). Further layers of endocuticle are deposited by the epidermal cells on the inner surface of the cuticle for days and even weeks after the molt has taken place.
Some elements of amphibian metamorphosis, namely, destructive processes (resorption of the old cuticle, necrosis of part of the epidermal cells) as well as constructive processes (re-arrangement of epidermal cells, formation of new cuticle), are present in an ordinary molt in insects. It depends on the condition of the epidermal layer whether the new structures produced during the molt are similar to or different from the old ones. In the first case the molt contributes to the growth of the animal; in the second, it becomes a mechanism for progressive development.
ADVERTISEMENTS:
If the changes achieved after a molt are considerable, the result is metamorphosis. In the primarily wingless archaic insects, the Apterygota, the young insect emerging from the egg is essentially similar to the adult, differing only in size and in the immature state of the sexual organs. Molting in these insects leads only to growth, and the advent of sexual maturity is not related in any way to molting, in fact, molting and growth continue even after the attainment of reproductive ability.
In all other insects, the Pterygota (winged or secondarily wingless forms), there is a distinct imaginal stage, which is attained after a specific imaginal molt, after which the insect does not molt any more. Except for secondarily wingless insects, the imaginal stage differs from the larval stages by the presence of wings. The imago also differs from the larval stages by the full development of external genital organs. (The gonads, on the other hand, may become fully functional only some-time after metamorphosis.)
In the more primitive winged insects, the wings appear gradually, the rudiments of the wings in the form of flat outgrowths of the second and third thoracic segments being visible in the later larval, or as they are often called, nymphal stages.
These rudiments increase with every subsequent molt, but at the last imaginal molt there is an abrupt and very marked increase in the size of the wings, and after this molt the wings become functional. (Only in the mayflies, the first winged stage, the sub-imago, molts again before it turns into an imago.)
The insects in which the rudiments of the wings develop on the surface of the body are called Exopterygota; these comprise the locusts, cockroaches, dragonflies, mayflies, bugs, and other related groups. In the most advanced orders of insects, however, the wings develop internally, as folded appendages concealed during the larval stages in deep pockets (infoldings) of the epidermis.
The epidermis covering these wing rudiments retains an embryonic character throughout larval life, and although the rudiments continue growing slowly, their epidermis does not participate in the formation of the external cuticle of the larva and comes into action only when the larval stage is drawing to an end. Such rudiments, concealed under the surface of the body in the larval stage and reaching full differentiation in the imago, are called imaginal discs. The insects in which the wings develop internally as imaginal discs are called Endopterygota; here belong caddis flies, beetles, butterflies, bees and wasps, mosquitoes and flies.
Although the development of the wings in the adult insect attracts the greatest attention, the other parts of the body also change at the time of metamorphosis from a larva or nymph to the adult (imago). Even where the larva or nymph leads the same mode of life as the adult and has a fairly similar general appearance, as in the locusts or bugs, many finer features of structure change.
In the bug, Rhodnius prolixus, for instance, which has been studied by Wigglesworth (1954), the fine structure of the cuticle and the pigmentation of the adult bug are very different from those in the last larval stage, so that even small areas of skin of the larva and the adult can be easily distinguished.
In the endopterygote insects, the difference between the larvae and adults is much greater. Not only wings but also mouth parts, antennae, and legs may be developed from imaginal discs, while the larval appendages become discarded, as in the case of butterflies.
ADVERTISEMENTS:
In some parasitic wasps and in flies such as Drosophila and Musca, the whole larval epidermis is discarded and replaced by the imaginal epidermis derived from a series of imaginal discs. Concurrently with the formation of appendages and other external parts, the internal organs are also reorganized.
As the locomotion of the winged adult is so completely different from the locomotion of a crawling larva, the muscle system may have to be radically changed. During metamorphosis of higher insects, the larval muscles become broken down, and their remnants are consumed by phagocytes. The adult muscles, in particular the muscles operating the wings (the flight muscles), are then developed.
The eyes of adult insects in the more advanced orders are quite different from those of the larvae and are developed from special imaginal discs. Most of the cells of the alimentary canal of the larva may undergo resorption, and the alimentary canal of the imago is lined by a new epithelium produced at the expense of pockets of small reserve cells, which are found between the functioning cells of the larval intestine.
While the moderate amount of transformations that occur in exopterygote insects can be performed in one molt, the reorganization that is needed to produce the imago in most endopterygote insects is so profound that a resting stage, the pupa, is intercalated between the larval and the adult condition.
In the pupa, the pockets containing the imaginal discs—wings, limbs, antennae, etc. — are brought to the surface. Internally, the formation of adult parts is, however, not yet completed, and while the reorganization takes place in the pupal stage, the insects do not take food and are very restricted in their movements, if they move at all.
When the reorganization is completed, another molt takes place, and the imago emerges from the pupa. Metamorphosis which includes a pupal stage is called complete metamorphosis, and the insects having this type of metamorphosis are called Holometabola.
Those not possessing a pupal stage and thus having an incomplete metamorphosis are Hemimetabola. The holometabolous insects are the Endopterygota; the terms, as far as systematics is concerned, are synonyms, though they stress different properties of the same group of insects.
Causation of Molting in Insects:
In an ordinary molt (larval molt) all parts of the body must participate in the process and carry it out at the same time, if the molting is to be successful. This suggests a common cause to which all parts of the insect are subjected. The existence of a common cause is even more obvious in the case of metamorphosis in which the involvement of both external and internal organs may be more far-reaching and radical. This common cause may be expected to be either external or internal.
Cases are actually known in which, under natural conditions, an external factor is necessary to start a molt. In the blood sucking bug, Rhodnius, such a factor is the intake of food. The bugs of this species feed only once in the interval between two molts, taking up so much blood that their body weight may increase many-fold.
Molting occurs regularly, 12 to 15 days after a feed in the case of the first four larval stages. The same dependence of molting on food intake holds true for the last, the fifth, larval stage, only the interval is somewhat longer, about 28 days, and the result is different – the molt transforms the larva into a winged imago.
Another example in which an external factor is necessary to initiate a molt is the case of the pupa of the moth Platysamia cecropia. After pupation, the insect falls into a quiescent state with a reduced rate of metabolism—the diapause— which continues throughout winter. It is essential that during this time the pupa be exposed to cold; otherwise the diapause is prolonged indefinitely.
However, the diapause may be broken precociously if the pupa is treated with cold (3° to 5°C.) for at least two weeks. The temporary cooling activates the vital processes in the pupa, and on return to a warmer environment the pupa molts, and in this way the development is completed with the emergence of the imago.
In the overwhelming majority of insects, however, no external cause of any molt can be found, and the molts follow one another at intervals which appear to be determined entirely by internal processes in the animal. In many insects the body weight increases in a fixed proportion between two molts, often by a factor of two, and it would appear that a certain amount of synthesis has to be performed after each molt before the stimulus for a new molt is generated in the organism.
However, even in cases in which an external factor triggers off the mechanism of molting, it can be shown that the factor in question does not affect all parts of the body directly but that it is mediated by the brain of the insect. If a larva of Rhodnius is decapitated within a day or two after feeding it does not molt, although it may remain alive for over a year. If, however, it is decapitated five or more days after a meal, molting takes place. By that time a stimulus generated by the brain reaches beyond the level of decapitation and is able to spread throughout the body and cause the molt to proceed.
A corresponding experiment in the moth Platysamia consists in activating one pupa by exposure to cold and then transplanting parts of the body of the activated pupa into an untreated pupa. The transplantation of the brain but not of other organs will cause the second pupa to molt and the adult moth to emerge, thus showing that the cold directly affects only the brain but that the rest of the body is stimulated to molting through the mediation of the latter.
The question naturally arises as to how the brain affects the rest of the body. It is now established that, as with amphibian metamorphosis, molting and metamorphosis in insects are controlled by hormones and that at least three organs of internal secretion are involved – the brain (proto-cerebrum), the corpora allata, and the prothoracic gland. In the brain, a hormone is produced by neuro-secretory cells, which are arranged in four groups – two groups near the midline and one group on each side.
Behind the proto-cerebrum, alongside the dorsal aorta, there are in most insects two pairs of bodies connected by nerve strands to the proto-cerebrum – first the corpora cardiaca, which are of the nature of nerve ganglia, and more posteriorly, the corpora allata, consisting of secretory cells. The corpora allata may be fused into one body in some insects. The third endocrine gland, the prothoracic gland, is an irregular branching mass of glandular cells located in the thorax, in close association with the tracheal tubes.
The glandular cells of all three centers show regular secretory cycles preceding each molt, and the three types of secretion are necessary for the normal course of larval molts. The molt is initiated by the neuro-secretory cells of the proto-cerebrum, but all that the hormone of the proto-cerebrum does is to activate the prothoracic gland.
The prothoracic gland then produces a hormone which sets in motion the mechanism of molting in the epidermis – the growth and proliferation of epidermal cells, the shedding of the old cuticle, and the production of the new one. The hormone produced by the prothoracic gland is therefore called the growth and molting hormone or ecdysone.
We have described some of the evidence proving that the initial stimulus for molting is given off by the brain (or the neuro-secretory cells of the proto-cerebrum). One of the experiments consisted in transplanting the brain of an activated pupa of Platysamia into an untreated pupa, whereupon the latter molted and produced the moth. A variation of the same experiment has been used to prove that the secretion of the brain cannot act directly but only through the activation of the prothoracic gland.
Instead of transplanting the activated brain into a whole pupa, it was implanted into the posterior half of a pupa which had been cut in two (the cut surface was sealed with paraffin wax). Under these conditions the graft was powerless; no metamorphosis took place. The reason for this is that the prothoracic gland is absent in the posterior half of the pupa. If in addition to the brain the prothoracic gland was also grafted, metamorphosis occurred. An analogous experiment has been performed on the bug Rhodnius.
After the neuro-secretory cells of the brain had been activated by the bug’s having a meal of blood, the brain was transplanted into the abdomen of a decapitated specimen. A decapitated larva is still in possession of the prothoracic gland, which could react to the implanted brain and cause the molt to proceed. If, however, the activated brain was implanted into an isolated abdomen, no molting occurred. On the other hand, molting could be induced in the isolated abdomen by the implantation of a prothoracic gland.
The roles of the brain and the prothoracic gland as causative agents of molting can also be demonstrated in insects in which the time of molting is not dependent on any particular external factor. If the brain is removed from caterpillars sufficiently early before the next expected molt, the caterpillars may remain alive for over two months but do not molt and do not pupate. The implantation of a brain from another caterpillar restores the ability of a brainless caterpillar to complete its development.
Once the prothoracic gland has become activated, the brain is no longer necessary for initiating the molt. Only those parts molt (or pupate), however, to which the hormone of the prothoracic gland has been able to gain access. If a caterpillar in the last larval stage is constricted behind the thorax, the anterior part of the body will pupate, but the posterior part, which the molting hormone could not reach, remains in the larval state. A little later, when the hormone has already spread throughout the body, a transverse constriction does not prevent pupation of the posterior end of the caterpillar.
The hormones emitted by the proto-cerebral neuro-secretory cells and the prothoracic gland induce an insect to molt, but they do not determine whether it will be a larval molt, producing the next larval stage, the pupal molt, converting the larva to pupa, or the imaginal molt, leading to the eclosion of the imago.
The third endocrine gland, the corpora allata, controls the nature of the change that takes place at the time of molting. Curiously enough, the first two glands, the proto-cerebral neuro-secretory cells and the prothoracic gland, when acting alone cause immediate metamorphosis—the development of the imago in hemimetabolous insects or of the pupa in holometabolous insects.
It is possible to remove the corpora allata from caterpillars of moths. Independently of the stage in which the operation is performed, the caterpillars proceed to pupate at the next molt. Eventually, the moth emerges from the pupa, although it may have reached only a fraction of the normal size. Apparently the presence of the corpora allata is necessary to prevent metamorphosis, to keep the insect in the larval state.
Accordingly, the secretion of the corpora allata has been called the juvenile hormone. The cells of the corpora allata show signs of secretory activity (swelling of cells, appearance and discharge of vacuoles, etc.) at every larval molt, but no such activity is present during the pupal or imaginal molt. Accordingly, it would seem that at the time metamorphosis takes place the corpora allata do not produce their secretion or at least are less active.
That it is actually the absence of the juvenile hormone that is the necessary condition for metamorphosis can be proved by implanting corpora allata from a young larva into the last stage larva which should be metamorphosing with the next molt. The larva may molt in due course, but under the influence of the juvenile hormone secreted by the graft, it is not transformed into an imago (in the case of a hemimetabolous insect) but instead produces an abnormally large larva.
In the case of holometabolous insects, the conditions are more complicated inasmuch as there are two molts accompanied by profound morphological changes—the pupal molt and the imaginal molt. The removal of the corpora allata from a caterpillar causes the caterpillar to be transformed into a pupa.
Some experiments, indicate that the subsequent transformation of the pupa into the imago is probably connected with a further decrease of the juvenile hormone in the blood of the insect. After the ablation of the gland, small amounts of the juvenile hormone could have still been present in circulation but would have been used up by the time of the second molt.
Factors Controlling Metamorphosis of Insects:
The agents produced by the prothoracic gland and the corpora allata are hormones, that is, chemical substances emitted by the cells and circulating in the body fluids. This could be deduced from the fact that the effect of the gland does not depend on whether it is in its normal position, with all its connections to neighboring organs and to the nervous system intact, or on whether it has been transplanted to an abnormal site. Further evidence in favor of a diffusible substance being the means of action of the gland will now be presented.
An equivalent of the glands’ independence of their positions is the independence of the reaction from the position of the reacting organs. When molting or metamorphosis occurs, not only do all parts of the body of the intact animal react together, but also transplanted parts do the same. Imaginal discs and other parts of the body may be transplanted between animals in different developmental stages, and they always molt and metamorphose together with the organs of the host and independently of their own age.
A very elegant experiment of this kind, carried out on the developing moth Ephestia kuhniella, consists in transplanting pieces of skin into the body cavity of another individual. The edges of the implanted piece of skin curl so as to form a cyst, with the original distal surface of the skin turned inward. The proximal surface of the epidermis is bathed by the body fluids of the host and by the host’s hormones if any are present in the body fluids.
The necessary conditions are thus provided for the graft epidermis to react to any hormones circulating in the body of the host. It was found that with every molt of the host the cyst epidermis molted also, discarding the old cuticle into the cavity of the cyst. Not only was the molting of the graft simultaneous with that of the host, but the nature of the new cuticle was always the same as that of the host.
When a larval molt occurred, the cyst epidermis produced a thin cuticle like the one that covered the body of the caterpillar. When the host pupated, the cyst produced a thick pupal cuticle. When the host metamorphosed into the adult moth, the epidermis of the cyst developed an imaginal cuticle with scales! All the successive cuticles could be seen later one inside the other, on sectioning the cyst.
Even after reaching the stage of producing the cuticle of the adult moth, the epidermis does not lose its capacity for molting, provided that ecdysone (the molting hormone) or both ecdysone and the juvenile hormone are present in the surrounding fluid. A cyst which had gone through the pupal and imaginal molts may be excised from the first host and transplanted into a second one.
If the second host is a caterpillar, the cyst will undergo a new molt simultaneously with the pupation of the host, will shed the imaginal cuticle with scales, and will again produce a thick pupal cuticle. This may be followed, at the time of metamorphosis of the host, by a second imaginal cuticle with a new set of scales.
Apparently metamorphosis is fully reversible, at least with regard to the skin epidermis, and the nature of differentiations produced by the latter is solely dependent on the balance of hormones present in the blood. A reversal of metamorphosis, even a partial one, can occur, however, only under experimental conditions. In the normal life of an insect, metamorphosis marks the end of morphogenesis and of growth (except for the growth of the gonads, which may continue in the adult).
The reason for the cessation of further development is that the prothoracic gland degenerates and breaks up after causing the last (imaginal) molt. With the prothoracic gland gone, no other factors can reawaken the morphogenetic activity of the epidermis, and no further molting can occur. This is also an explanation for the existence of a growth limit in insects.
The action of the juvenile hormone is not restricted to the control of qualitative changes in the body of the insect, but it appears to have also a direct influence on growth. It is found that in the imago, after metamorphosis, the corpora allata resume their secretory activity and that their secretion is necessary for the growth of the ovaries and the oocytes.
In vertebrates, the chemical nature of the agents emitted by the endocrine glands has been proved by preparing active extracts from the glands, containing chemically definable substances. The extraction of hormones from insects is much more difficult, owing to the small mass of the endocrine glands. However, some success can be noted in this field also.
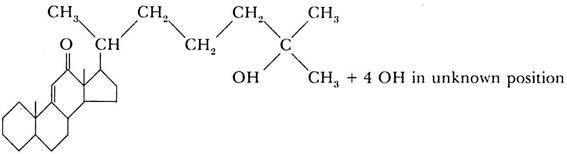
The molting hormone, ecdysone (the hormone of the prothoracic gland), has been prepared in a chemically pure form by Butenandt and Karlson (1954). Five hundred kilograms of silkworm pupae were used to isolate 25 mg. of the pure substance, and 0.0075 mg. of this material was sufficient to cause the pupation of a fly larva. The chemical analysis of the substance gave the empirical formula C27 H44 O6.
The substance belongs to the steroid group, and its approximate structural formula is:
The chemical analysis of the other two hormones, the brain hormone and the juvenile hormone, has not yet been as successful. The results obtained by different research workers are somewhat contradictory. It appears, however, that the juvenile hormone is chemically related to ecdysone and is also a steroid or a substance of the terpene group which is a possible precursor of steroids.
As in the case of inducing substances in early amphibian embryogenesis, it has been found that the action of the juvenile hormone may be imitated by extracts of tissues of various animals and by certain substances of known chemical composition, such as the terpene farnesol.
The latter is also able to stimulate the prothoracic gland, an effect normally produced by the brain hormone. It has been suggested, therefore, that all three hormones regulating growth and development in insects have something in common in their chemical structure and activities.
Mechanism of Action of Insect Hormones:
The study of insect molting and metamorphosis has contributed some very interesting information on the way in which chemical substances act on cell differentiation.
It has been known for many years that in the salivary glands of some insects in the order Diptera certain cells grow to a relatively large size, and that in such cells the chromosomes become visible, although the cells do not undergo mitosis.
The “giant chromosomes” in such cells are the result of repeated duplication of the DNA molecules, so that many hundreds of DNA molecules lie side by side. Cells of malpighian tubules and some other tissues also grow excessively and produce giant chromosomes. The chromosomes show characteristic transverse bands which may be related to specific genes as known from breeding experiments.
Even before the relation of the bands on the giant chromosomes to genes was known, conspicuous thickenings were found on some giant chromosomes. These thickenings were known for many years as the “Balbiani rings,” so named after the scientist who discovered them first in salivary chromosomes of the midge Chironomus. Later, it was found that the thickenings occur in all cases where giant chromosomes are found, though not all are as large as the Balbiani rings. The thickenings, large or small, are now called puffs.
A puff is actually a section of the chromosome in which the numerous strands of DNA, of which the giant chromosome consists, become separated from one another and form loops, extending outward from the main axis of the chromosome. This loosening of the chromosomal structure is favorable for the chemical interaction of the DNA strands with their environment inside the nucleus. In fact, it has been proved (by using radioactive precursors) that there is a very rapid synthesis of RNA on the puffs.
The RNA synthesized on the puffs is different in base composition from the cytoplasmic ribosomal RNA and is believed to be messenger RNA. In other words, the puffs represent sections of chromosomes in which the genes are in an “activated” state and are particularly active in producing messenger RNA.
A further important discovery was made that the pattern of puffing changes with the stage of development of the insect; puffs appear at different points on the chromosomes and may disappear again at a later stage, while new puffs develop at other points. The stage of metamorphosis is characterized by a specific pattern of puffs which can be recognized if the structure of the giant chromosomes of a species under investigation is known in detail.
The question now arises as to whether there is a connection between the structural changes in the chromosomes and the morphogenetic changes (molting, metamorphosis) of the whole animal. A priori one could expect that changes in the activity of genes must bear some relation to the transformations of the animal’s organization. On the other hand, we have seen that molting and metamorphosis in insects is under the control of hormones (ecdysone and juvenile hormone).
A breakthrough in this field was achieved when it was shown that injection of pure ecdysone into Chironomus larvae caused the giant chromosomes to acquire a puffing-pattern identical to the one occurring during pupation.
The changes in the chromosomes occurred very rapidly; the first reactions were visible within 15 to 30 minutes after the injection, whereas the changes in the cuticle of the larvae leading to pupation occurred in a matter of a few days. It seems reasonable to conclude that the hormone acts initially on the genes and that the changed activity of the genes then causes a changed behavior of the cells and tissues.
Evidence has been obtained to show that different loci on the chromosomes do not react to ecdysone in the same way. There are one or two loci which produce puffs very early after ecdysone injections and appear to be the immediate reacting sites to the hormone.
Other loci, in which puffs may arise or increase in size later, are claimed to be dependent on the action of the genes activated in the first place. In this way it would appear that the action of ecdysone may consist in activating only two or possibly even only one gene, and that this starts a chain reaction, involving activities of other genes, which eventually ends in molting or metamorphosis.
Doubts have been expressed, however, whether ecdysone as such acts on the gene or whether it changes the intra-nuclear milieu, which, in turn, then affects the gene. It was found that the appearance of puffs on giant chromosomes can be invoked by means other than specific hormone action. Changes in ion concentration, certain narcotics, and even the simple fact of explanting the salivary gland in hemolymph may influence the puffing pattern of the chromosomes.
The changes may be similar to those occurring in the chromosomes under natural conditions at certain periods of development. It has been claimed; in particular, that explantation of salivary glands in hemolymph causes the chromosomes to revert to a puffing pattern which is similar to that found in earlier stages of development (“rejuvenation”). Some narcotics and ZnCl2 in particular, when administered at a sensitive early pupal stage may produce a pattern of puffing characteristic of a later stage of pupation.
The puffs caused by ZnCl2 show an increased incorporation of RNA precursors, but it has not been shown that the unspecific agents, can cause metamorphosis or molting, nor indeed any morphological effect except on the chromosomes.
It is interesting to note the close similarity between the results obtained in experiments on insect metamorphosis and the conclusions which emerge from work on the primary organizer (neural inductor) in amphibian embryos.
In both cases, the obvious reaction involves the activities of groups of cells, which change their behavior and acquire new differentiations. (In insects, cells change from the larval condition to that of a pupa or an imago; in an amphibian embryo, gastrula ectoderm becomes differentiated as neural tissue.)
In both cases we have been led to the conclusion that the change is produced by a modification of the activity of nuclear genes. This modification is not a spontaneous change in the genes themselves but is evoked by an external factor-ecdysone in the metamorphosing insect, neutralizing substance in the amphibian embryo.
Lastly, the action of the natural inducing substances may in both cases be imitated by “abnormal inductors,” though in the case of insect metamorphosis and molting the “abnormal inductors” have not been able to copy the effect of the natural agents in their entirety.