The following points highlight the four major disorders of aldosterone secretion. The disorders are: 1. Primary Aldosteronism 2. Glucocorticoid-Suppressible Aldosteronism 3. Hyper-Aldosteronism Due to 17- Hydroxylase Deficiency 4. Syndrome of Apparent Mineralocorticoid Excess.
Disorder # 1. Primary Aldosteronism:
Primary aldosteronism or Conn’s syndrome results from increased production of aldosterone by adrenal zona glomerulosa. Cells of this zone do not have the ability to make Cortisol owing to the absence of P450C17, 17α-hydroxylase enzyme systems.
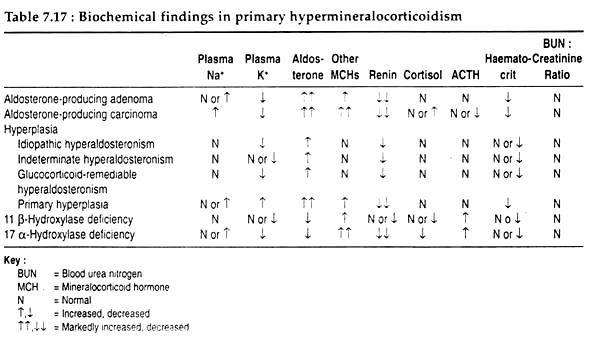
Thus, there are normal Cortisol production and metabolism. Excess mineralocorticoid production may result from other causes and their physiological consequences are listed in Table 7.17.
Pathogenesis and symptoms:
ADVERTISEMENTS:
The over- secretion of aldosterone leads to increased sodium retention that, in turn, results in expansion of extracellular fluid (ECF) volume and increased total body sodium content. In addition to kidney, fecal excretion of sodium is decreased.
The expanded ECF and plasma volumes are registered by stretch receptors at the juxtaglomerular apparatus and sodium flux at the macula densa, with resultant suppression of renin secretion. These effects of aldosterone are further manifested by normal or elevated serum sodium concentration and reduced haematocrit.
In addition to sodium retention, potassium depletion also develops. The extrusion of potassium from its intracellular reservoir is followed by the intracellular movement of hydrogen ion, and increased renal secretion of hydrogen ion-ensues alkalosis. Consequently, decreased carbohydrate tolerance and resistance to antidiuretic hormone (vasopressin) occur.
ADVERTISEMENTS:
In spite of these physiological disorders, excessive production of mineralocorticoids produces no characteristic physical findings. Blood pressure in patients with primary aldosteronism can range from borderline to severe hypertensive levels.
Postural falls in blood pressure without reflex tachycardia, are observed in the severely potassium depleted patient because of blunting of the baroreceptors. The heart is mildly enlarged and clinical edema is rare.
Disorder # 2. Glucocorticoid-Suppressible Aldosteronism (GSA):
In this rare form of hyper-aldosteronism, aldosterone secretion is regulated by ACTH. It is likely that during the transition from one type of cortical cells to another, intermediate cell type(s) are formed.
This intermediate cells are able to synthesise Cortisol and aldosterone, as well as the hybrid steroids (18-hydroxy-cortisol and 18- oxocortisol), having both 17 and 18 hydroxylation by the action on Cortisol of the same enzyme(s) responsible for the final steps of aldosterone synthesis.
ADVERTISEMENTS:
The hyper-secretion of aldosterone and consequent elevation of blood pressure, however, can be corrected by chronic glucocorticoid therapy.
Disorder # 3. Hyper-Aldosteronism Due to 17- Hydroxylase Deficiency:
Malfunction of 17- hydroxylase activity results in deficient biosynthesis of Cortisol and androgens. With diminished activity in 17-hydroxylase function, adrenal metabolism is shunted toward increased production of aldosterone, as well as the production of mineralocorticoid precursors, like 11-deoxycorticosterone and corticosterone.
Absence of glucocorticoid feedback to the brain results in enhanced ACTH secretion and an aggravated production of precursor to mineralocorticoid synthesis. The resultant hypertension, however, can be alleviated by dexamethasone administration which feeds back to the hypothalamus, resulting in decreased ACTH secretion.
Disorder # 4. Syndrome of Apparent Mineralocorticoid Excess:
Some symptoms of primary aldosteronism with hypertension, hypokalemic alkalosis and suppressed renin activity are observed in children and young adults. It is likely that the functioning mineralocorticoid in this disorder is Cortisol, circulating in normal amounts, but exerting a mineralocorticoid effect because of incomplete metabolism at target tissues.
This abnormality in steroid metabolism in this syndrome appears to be due to a decreased intracellular conversion of Cortisol to cortisone—which itself is biologically inactive.