In this article we will discuss about:- 1. Structure of Adrenal Gland 2. Development of Adrenal Gland 3. Location 4. General Anatomy 5. Histology and Cytology 6. Hormones.
Structure of Adrenal Gland:
The adrenal gland is a compound structure and, in mammals, it consists of an outer cortex and an inner medulla. The cortex and medulla are both endocrine glands, but they differ in their embryological origin, and their secretions are under different modes of control.
Nevertheless, the hormones of both parts play important roles in response to stress. The adrenal glands are indispensable for normal physiological activity. Their removal or destruction by disease is followed by death of the animal.
Development of Adrenal Gland:
A. Adrenal cortex:
ADVERTISEMENTS:
The adrenal cortex is of mesodermal origin and is derived from lateral mesoderm in close association with the developing gonads.
B. Adrenal medulla:
The medulla differentiates from neural crest cells along with the sympathetic ganglia. At about 6th week of gestation, groups of primitive cells of neural crest migrate along the central vein and enter the foetal adrenal cortex to form the adrenal medulla.
Location of Adrenal Gland:
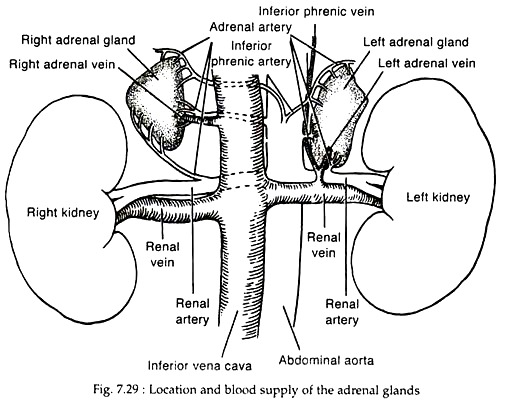
The adrenal glands are embedded in adipose tissue. It lies in the retro peritoneum above or medial to the upper poles of the kidneys (Fig. 7.29).
General Anatomy of Adrenal Gland:
The adrenal glands in human are relatively flat triangular organs, less than 1 cm in thickness, and ranging in width from 2 cm at the apex to 5 cm at their base. In adult their combined weight is 15 to 20 gm. A fibrous capsule surrounds the gland; the yellowish outer cortex comprises 90%, while the inner medulla about 10% of the adrenal weight.
The cortex is richly vascularized and receives its main arterial supply from inferior phrenic artery, renal arteries and the aorta. The branches of these arteries form an arterial plexus beneath the capsule and then enter a sinusoidal system that penetrates the cortex and medulla; draining into a single central vein in each gland. The right adrenal vein drains into the posterior vena cava, whereas the left vein enters the left renal vein (Fig. 7.29).
In mammals, the medulla is surrounded by the cortex. In human, the medulla occupies a central position in the widest part of the gland, with only small portions extending into the narrower parts. There is no clear demarcation between cortex and medulla.
The central vein is usually surrounded by cuff of adrenal cortical cells and there may be islands of cortex elsewhere in the medulla. The cells of the adrenal medulla are innervated by preganglionic fibres of the sympathetic nervous system. Some of such fibres reach the wall of the central vein, where they synapse with small autonomic ganglia.
ADVERTISEMENTS:
The medulla is nourished by some vessels that supply the adrenal cortex. A few of such vessels penetrate the cortex, passing directly to the medulla. Arteries supplying the central vein and cuff of cortical tissue around the central vein also supply the medulla. It would appear that most of the blood supply to the medullary cells is via a portal vascular system arising from the capillaries in the cortex.
Histology and Cytology of Adrenal Gland:
(i) Adrenal Cortex:
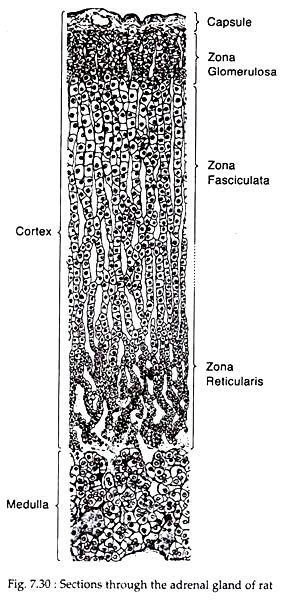
Histologically the adult human adrenal cortex is composed of three concentric zones:
(i) A thin, outer zona glomerulosa immediately beneath the capsule;
(ii) A broad, intermediate zona fasciculata
(iii) An inner zona reticularis adjacent to the medulla (Fig. 7.30).
In human, these three zones constitute, respectively, 15%, 78% and 7% of the volume of the cortex. The transition from zone to zone appears to be gradual in routine histological sections.
(i) Zona glomerulosa:
ADVERTISEMENTS:
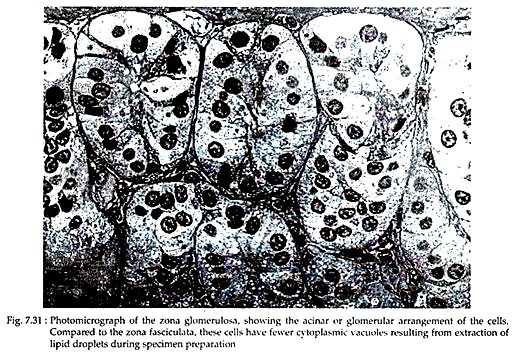
In this outer cortical zone, columnar epithelial cells form closely spaced arcades that bear a superficial resemblance to the acini of exocrine glands. These are separated by thin connective tissue septa that extend inward from the capsule. The cells have deeply stained nuclei with one or two nucleoli and the cytoplasm contains scattered clumps of basophilic materials (Fig. 7.31).
In electron micrographs, the cells of zona glomerulosa appear to bear a few short, irregularly oriented microvilli where exposed to the interstitium. The nuclei of the outer cells are often irregular in outline, whereas those deeper in the zone are spherical.
The Golgi complex is relatively small and a network of smooth endoplasmic reticulum (SER) is found throughout the cytoplasm; but SER and lipid droplets are less extensively present in these cells than in cells of other cortical zones.
(ii) Zona fasciculata:
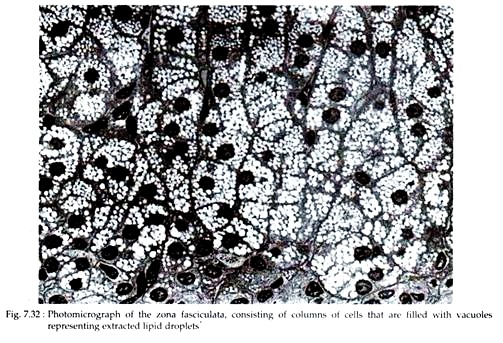
The middle cortical zone is made up of pale staining polyhedreal cells arranged in long columns that are oriented radially in relation to the medulla. The columns are one or two cell thick and separated from one another by sinusoids or capillaries of similar prevailing orientation. The acidophilic cytoplasm of the cells often shows highly vacuolated appearance owing to the extraction of abundant lipid droplets during the preparation of the specimen (Fig. 7.32).
In electron micrographs, the cells are larger than those of glomerulosa and the cytoplasm contains large numbers of lipid droplets of low electron density occupying a large volume of the cytoplasm. A very extensive smooth endoplasmic reticulum that occupies 40-45% of the cell volume is the most distinctive feature of fasciculata cells.
The juxta nuclear Golgi complex is unremarkable and mitochondria are shorter than those of zona glomerulosa. The nucleus has a prominent nucleolus and a distinct fibrous lamina. Most of the cytological features varies from species to species.
(iii) Zona reticularis:
The parallel cell columns of zona fasciculata give way to a three-dimensional network of anastomosing cell cords in the inner zona reticularis. The cells of this zone are smaller and stain more deeply due to less number of extracted lipid droplets in the cytoplasm than the cells of the fasciculata.
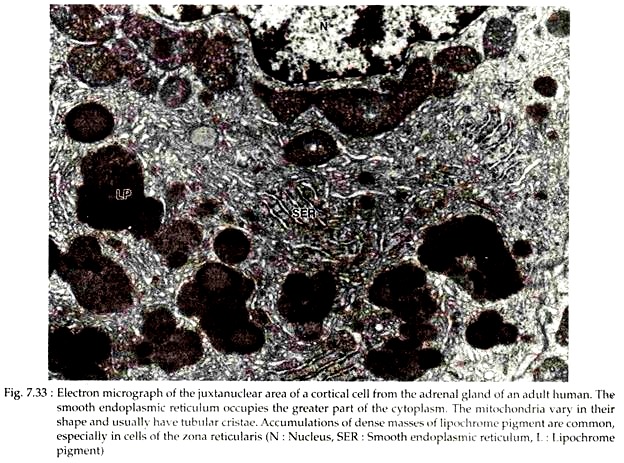
The electron micrographs of these cells reveal the presence of far less extensive endoplasmic reticulum, small Golgi complex and few lipid droplets in the cytoplasm. Large aggregations of lipochrame pigment are common (Fig. 7.33). Near the medulla there are variable numbers of “dark cells” with more electron-dense cytoplasm and shrunken hyper-chromatic nucleus. These are degenerating cells.
(ii) Adrenal medulla:
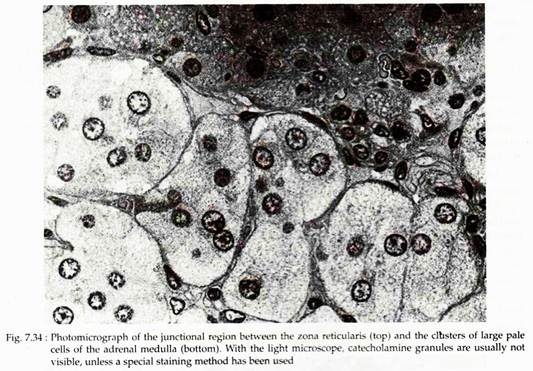
In human, the boundary between the cortex and medulla of adrenal gland is irregular with some reticularis cords penetrating a short distance into the medulla. In the medulla, large epitheloid cells are arranged in rounded clusters or short cords that are in intimate relation to capillaries and venules (Fig. 7.34). These cells in histological preparations, fixed with solution containing potassium dichromate (chromic acid) show numerous small brown granules.
Such cells and isolated groups of cells in other organs that display this staining reaction are called chromaffin cells or pheochromocytes (pheo-brown). The browning of granules with chromium salts is called chromaffin reaction that is due to oxidation and polymerisation of catecholamine present in the granules. The medulla can be thought of as a modified sympathetic ganglion made up of postganglionic cells that lack dendrites and axons.
In medulla two kinds of chromaffin cells can be distinguished by histochemical reactions. Cells that store norepinephrine have a low affinity for the dye azocarmine, are auto-fluorescent and are negative for acid phosphatase. On the other hand, epinephrine storing cells have a high affinity for azocarmine, are not fluorescent and show a positive acid phosphatase reaction.
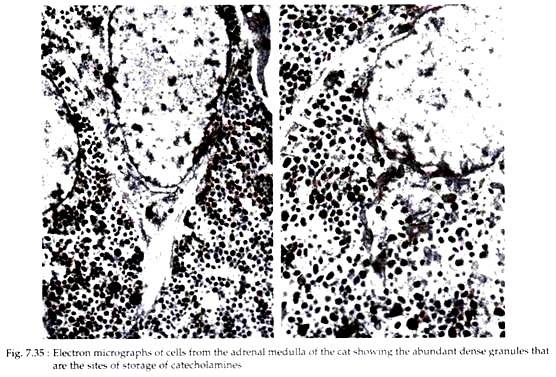
In electron micrographs of adrenal medulla, the most prominent feature of the cells is their content of large numbers of small membrane bound dense granules (Fig. 7.35). These granules store catecholamines. Granules storing norepinephrine have an electron dense core that is often eccentric in its position within the limiting membrane.
Cells that store epinephrine have granules with a more homogeneous content of lower density. In isolated medullary granules, there remains a soluble protein called chromagranins together with ATP and enkephalins in addition to norepinephrine and epinephrine. It is suggested that catecholamines form high- molecular weight aggregates with ATP and divalent cations within the granules.
The cytoplasm of catecholamine bearing cells contains short parallel arrays of rough endoplasmic reticulum and a moderate number of mitochondria. Materials interpreted as precursor of secretory granules are often found in the trans-cisternae of Golgi complex.
Hormones of Adrenal Gland:
Hormones of adrenal cortex:
The major hormones secreted by the adrenal cortex are Cortisol (glucocorticoids), androgens (sex corticoids) and aldosterone (mineralocorticoids). Of these steroids, aldosterone is produced by zona glomerulosa. This layer lacks 17 α-hydroxylase activities and thus is unable to synthesise precursor of Cortisol and adrenal androgens.
The zonae fasciculata and reticularis produce Cortisol, androgens and little amount of estrogens. These zones do not contain the enzyme P450 aldo (aldosterone synthase) and, therefore, cannot synthesise aldosterone.
The overall process of adrenal steroidogenesis can be discussed as:
1. Cholesterol uptake and synthesis:
Synthesis of Cortisol and androgens by the zonae fasciculata and reticularis begins with cholesterol, as does the synthesis of all steroid hormones. The major source of adrenal cholesterol is plasma lipoproteins, though synthesis within the gland from acetate may also occur. A small pool of free cholesterol is also available for rapid synthesis of steroid.
The low density lipoprotein-cholesterol is transferred into the cell by endocytosis; the cholesterol splits from lipoprotein, esterified and is stored in cytoplasmic vacuoles. Stimulation by ACTH activates the cholesterol esterase, which releases free cholesterol from its storage depots, providing the substrate for steroid synthesis.
2. Cholesterol metabolism:
In the next step cholesterol is converted to pregnenolone. The substrate cholesterol is transferred from the outer mitochondrial membrane to the inner mitochondrial membrane, the site of cholesterol side chain cleavage. It is the rate-limiting step in steroid biosynthesis and is mediated by the steroidogenic acute regulatory protein (STAR), whose de novo synthesis is stimulated by ACTH.
The synthesis of various adrenal hormones involves cytochrome P450 enzymes, which are oxygenase of mixed functions that catalyse steroid hydroxylations. Cytochrome P450 side chain cleavage enzyme (P450 see) or 20, 22 desmolase of inner mitochondrial membrane first hydroxylates the side chain C22 and C20 of cholesterol successively, cleaving the side chain and changes cholesterol to pregnenolone. This side chain cleavage occurs in all three zones of adrenal cortex. Pregnenolone is then transported outside the mitochondria before further steroid synthesis occurs.
3. Synthesis of Cortisol:
In the cells of zona fasciculata, within smooth ER, pregnenolone is changed to 17 α-hydroxypregnenolone by 17 α-hydroxylase. This steroid is then converted to 17 α-hydroxy progesterone by the action of 3 β-hydroxysteroid di-hydrogenase: Δ5, 4 isomerase pair.
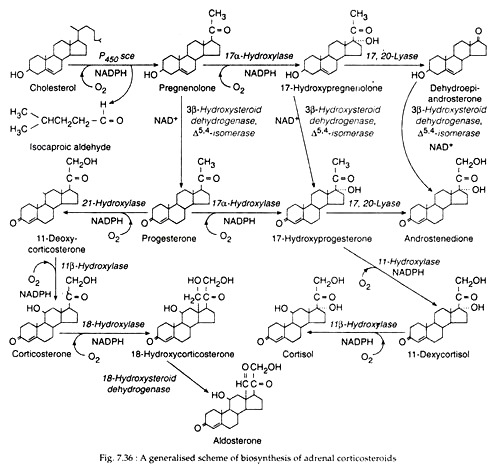
The next step, which is again microsomal, involves the 21-hydroxylation by P450 C21 of 17 α-hydro-xyprogesterone to form 11-deoxycortisol; this compound is further hydroxylated within mitochondria by 11 β-hydroxylation (P450C11) to form Cortisol (Fig. 7.36).
By a different sequence of actions of the same enzymes pregnenolone may also be changed to Cortisol through progesterone → 17 α-hydroxyprogesterone → 11 deoxycortisol → Cortisol (Fig. 7.36). A small amount of pregnenolone may be changed to another glucocorticoid, corticosterone by still another sequence of actions of the same enzymes (Fig. 7.36).
4. Synthesis of androgens:
In the cells of zonae fasciculata and reticularis, 17α-hydroxypregnenolone undergoes removal of its two-carbon side chain at the C17 position by microsomal 17, 20-desmolase, yielding DHEA (dehydroepiandrosterone) with a keto group at C17.
DHEA is then converted to DHEA sulfate by a reversible adrenal sulfokinase. The other major adrenal androgen, androstenedione is produced from 17α-hydroxyprogesterone by 17, 20-desmolase and to a lesser extent from DHEA (Fig. 7.36).
The C17-ketonyl oxygen of small amounts of androstenedione may be reduced by 17β- hydroxysteroid dehydrogenase to give rise to testosterone. Small amounts of progesterone may be formed from pregnenolone in the glomerulosa cells by the successive actions of microsomal 3β-hydroxysteroid dehydrogenase (3β HSD) and Δ5, 4 isomerase.
5. Synthesis of aldosterone:
The cells of zona glomerulosa do not possess microsomal 17α-hydroxylase. Therefore, on their smooth ER tubules, pregnenolone is converted successively to progesterone and 11- deoxycorticosterone by the actions of microsomal 3βHSD: A5, 4-isomerase pair, and 21- hydroxylase, respectively (Fig. 7.36).
11-deoxycorticosterone then transferred into mitochondria where the successive actions of 11β-hydroxylase and 18-hydroxylase convert it through corticosterone to 18-hydroxy-corticosterone. The latter is then oxidised to principal mineralocorticoid, aldosterone by 18-hydroxysteroid dehydrogenase (18 HSD) (Fig. 7.36).
Regulation of adrenal steroid synthesis:
(a) Glucocorticoids:
ACTH stimulates the synthesis and secretion of glucocorticoids by the fasciculata cells in several ways:
(i) ACTH enhances the transfer of cholesterol from plasma LDL into the fasciculata cells by increasing the number of LDL receptors on the plasma membrane.
(ii) ACTH stimulates cholesterol esterase which hydrolyses cholesterol esters of intracellular storage pools into free cholesterol. Thus it enhances the availability of free cholesterol for steroidogenesis.
(iii) The trophic hormone increases the binding of cholesterol to mitochondrial cytochrome P450 in the fasciculata cells for hydroxylation of cholesterol.
(iv) It activates the P450 side chain cleavage enzyme for the cleavage of side chain and subsequent conversion of cholesterol to pregnenolone. Thus, the rate limiting step in glucocorticoid synthesis is enhanced by ACTH in the cortical fasciculata cells.
ACTH is the trophic hormone of zonae fasciculata and reticularis and the major regulator of Cortisol secretion. ACTH in turn is regulated by the hypothalamus and central nervous system via neurotransmitters and corticotropin releasing hormone (CRH).
(b) Adrenal androgen:
Adrenal androgen production in adults is also regulated by ACTH, both DHEA and androstenedione exhibit circadian periodicity in concert with ACTH and Cortisol. Plasma concentrations of both the steroids increase rapidly with ACTH administration and are suppressed by glucocorticoid administration, confirming the role of endogenous ACTH in their secretion.
(c) Mineralocorticoids:
Synthesis and secretion of aldosterone is regulated mainly by plasma concentration of sodium and potassium ions (Na+, K+), renin-angiotensin system and, to some extent, corticotropin also. A rise in plasma K+ concentration stimulates aldosterone synthesis and secretion by activating some enzymes for aldosterone synthesis and by up-regulating angiotensin receptors in glomerulosa cells.
Low plasma level of Na+ stimulates aldosterone secretion by directly increasing its synthesis and by stimulating renin-angiotensin system. However, glomerulosa cells are less sensitive to Na+ depletion than to K+ excess.
Angiotensin II is formed as a result of Na+ depletion hypotension, or a rise to erect posture. It increases aldosterone synthesis by enhancing enzyme activities in converting cholesterol to pregnenolone and corticosterone to aldosterone. It also up-regulates the angiotensin receptors on glomerulosa cells.
Corticotropin is also suggested to augment the effects of angiotensin II and high plasma K+ on the glomerulosa cells. Thus, ACTH plays some minor role in aldosterone secretion. However, aldosterone secretion was found to increase after ACTH administration but decline only in a couple of days.
Hormones of adrenal medulla:
The adrenal medullary chromaffin cells synthesize two catecholamines, adrenaline or epinephrine (E) and noradrenaline or norepinephrine (NE). The latter is found not only in adrenal medulla in mammals, but also in central nervous system and in the peripheral sympathetic nerves. Dopamine, the precursor of norepinephrine, is found in high concentration in brain, carotid body, mast cells and entero-chromaffin cells. Norepinephrine usually acts as local hormone and is involved in neurotransmission.
Biosynthesis:
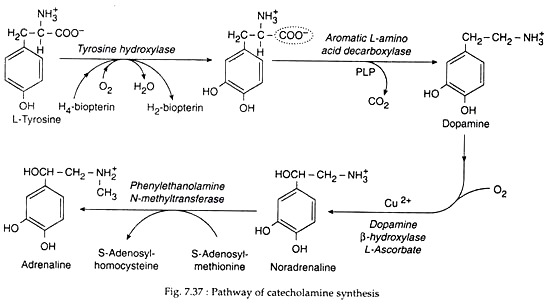
Catecholamines are synthesised from tyrosine. Its biosynthesis may be discussed as:
(1) Conversion of tyrosine to Dopa:
Tyrosine is either derived from ingested food or synthesised from phenylalanine in the liver. It enters neurons and chromaffin cells by an active transport mechanism and is converted to dihydroxyphenylalanine (DOPA). This reaction is catalysed by tyrosine hydroxylase, which is transported via axonal flow to the nerve terminal. This is the rate-limiting enzyme for catecholamine synthesis (Fig. 7.37).
(2) Conversion of Dopa to Dopamine:
Dopa is then decarboxylated to dopamine by PLD-dependent aromatic L amino acid decarboxylase (dopa decarboxylase). This enzyme is found in many tissues with highest concentration in liver, kidney, brain and vas deferens.
(3) Conversion of dopamine to norepinephrine:
The conversion of dopamine to norepinephrine is catalysed by dopamine β hydroxylase, a copper oxidase having mixed functions. This enzyme requires oxygen and an external electron donor. This enzyme does not occur in tissues outside the neuron. The Cu+ ion of the enzyme is oxidised to Cu2+ during catalysis (hydrolysis) of dopamine and is then reduced back to Cu+ by L-ascorbate.
(4) Conversion of norepinephrine to epinephrine:
Epinephrine is synthesised from norepinephrine by the action of the enzyme phenyl ethanolamine-N-methyltransferase (PNMT). PNMT catalyses the N-methylation of NE to E, using S-adenosylmethionine as a methyl donar.
The enzyme is found in the cytosol. NE after being synthesised from dopamine leaves the granules and after methylation re-enters different granules. The synthesis of PNMT is induced by Cortisol. As the adrenal medulla is cradled by the cortex, it is bathed with very high concentration of Cortisol, so that PNMT is available.
Storage and Release:
The catecholamines are stored in electron-dense granules that contain catecholamine and ATP in a 4:1 molar ratio, several neuropeptides, calcium, magnesium and water soluble proteins called chromogranins. The Mg2+-dependent ATPase facilitates the uptake and inhibits the release of catecholamines by the granules.
The release of catecholamines occurs by “stimulus-secretion coupling”. Preganglionic nerve impulses release acetylcholine, which increases membrane permeability, thus depolarizing the cell membrane and increases calcium uptake. This induces reverse pinocytosis of the secretory granules or exocytosis.
Regulation of catecholamine synthesis and secretion:
Acetylcholine and ACTH increase the synthesis of tyrosine hydroxylase while glucocorticoid enhances the activity of PNMT, thus enhancing the synthesis of catecholamine in chromaffin cells. Stresses, like hypoglycemia, haemorrhage, trauma and low temperature, enhance E and NE secretion by stimulating ACTH and glucocorticoid secretions. Acetylcholine also increases the release of catecholamine from chromaffin granules through Ca2+ dependent mechanism.
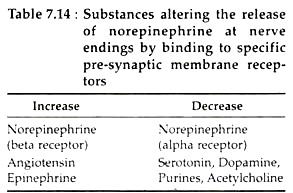
Norepinephrine has an important role in modulating its own release by activating the alpha receptors on the presynaptic membrane. Conversely, presynaptic beta receptors enhance NE release, whereas beta receptor blockers increase it. The effect of such substances are shown in Table 7.14