The following points highlight the two main types of disorders of adrenocortical functions. The types are: 1. Primary Adrenocortical Insufficiency 2. Secondary Adrenal Insufficiency.
Type # 1. Primary Adrenocortical Insufficiency:
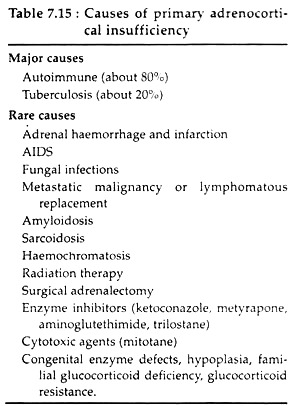
Primary adrenocortical deficiency leads to Addison’s disease which results from adrenocortical atrophy due to autoimmune, tubercular or malignant affliction of adrenal glands.
The various other causes are listed in Table 7.15:
Pathophysiology and Symptoms:
ADVERTISEMENTS:
In chronic primary adrenocortical insufficiency, gradual destruction of both cortices occurs. In this condition, basal steroid secretion is normal, but secretion does not increase in response to stress. Destruction of the adrenals by haemorrhage results in sudden loss of both glucocorticoid and mineralocorticoid secretion, accompanied by acute adrenal crisis.
With decreasing Cortisol secretion, plasma levels of ACTH and β-lipotropin are increased because of decreased negative- feedback inhibition of their secretion.
Low blood Cortisol causes a compensatory rise in pituitary lipotropin secretion with increased MSH activity, resulting in a bronze pigmentation of skin and mucosae (the earliest manifestation of Addison’s disease). Cortisol deficiency also causes weakness, fatigue, anorexia, nausea, hypotension and hypoglycemia.
ADVERTISEMENTS:
Mineralocorticoid deficiency produces renal sodium wasting and potassium retention and can lead to severe dehydration, hypotension, hyponatremia, hyperkalemia, acidosis and cardiac arrhythmia, muscular weakness etc.
In acute adrenal cry-sis hypovolemic shock frequently occurs; other symptoms include hyponatremia, hyperkalemia, lymphocytosis, eosinophilia and hypoglycemia. Shock and coma may often lead to death in untreated patients.
Type # 2. Secondary Adrenal Insufficiency:
Secondary adrenocortical insufficiency may result due to ACTH deficiency which, in turn, results from exogenous glucocorticoid therapy. However, pituitary and hypothalamic tumors may be the most common causes of naturally occurring pituitary hypo-secretion.
Pathophysiology and symptoms:
ADVERTISEMENTS:
ACTH deficiency leads to decreased synthesis of Cortisol and androgen secretion from cortex. Aldosterone secretion, however, remains normal except in a few cases. With loss of basal ACTH secretion, zonae fasciculata and reticularis show atrophy; and therefore, basal Cortisol secretion is decreased.
At this stage, the entire pituitary adrenal axis is impaired, i.e. there is not only decreased ACTH responsiveness to stress but also decreased adrenal responsiveness to acute stimulation with exogenous ACTH.
The symptoms of secondary adrenal insufficiency differ from that of primary insufficiency in that pituitary secretion of ACTH and P-LPH is deficient and hyperpigmentation is, therefore, not present. Moreover, mineralocorticoid secretion is usually normal, so volume depletion, dehydration and hyperkalemia are usually absent.
Hypernatremia may occur due to water retention and inability to excrete a water load but is not accompanied by hyperkalemia. Hypoglycemia is occasionally present. Acute de-compensation with severe hypotension or shock unresponsive to vasopressors may result.
Cushing’s syndrome:
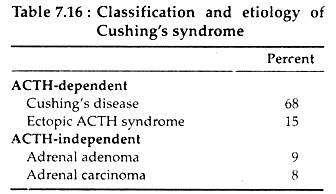
Chronic excess secretion of glucocorticoid leads to a constellation of symptoms and physical features known as Cushing’s syndrome. It may be ACTH dependent or ACTH independent (see Table 7.16).
The ACTH dependent types of Cushing’s syndrome (ectopic ACTH syndrome and Cushing’s disease) is characterised by chronic ACTH hyper-secretion, which results in hyperplasia of the adrenal zona fasciculata and zona reticularis and therefore, there is increased adrenocortical secretion of Cortisol, androgen and DOC.
ACTH independent Cushing’s syndrome is due to autonomous glucocorticoid secreting adrenocortical adenoma or carcinomas. In these cases, excess Cortisol secretion suppresses pituitary ACTH secretion.
ADVERTISEMENTS:
Pathophysiology and symptoms:
1. Cushing’s disease:
In Cushing’s disease, spontaneously arising corticotroph cell in pituitary adenomas cause the consequent ACTH hyper-secretion and hypercortisolism leading to characteristic endocrine abnormalities and hypothalamic dysfunction.
Here feedback inhibition of ACTH by physiological levels of glucocorticoid is absent, and thus, ACTH hyper-secretion persists despite elevated Cortisol secretion and results in chronic glucocorticoid excesses.
There is no diurnal variation in ACTH and Cortisol secretions. This leads to reduced sugar tolerance, hyperglycemia, glucosuria, negative N-balance, wasting, abnormal distribution of fats in some areas like ‘moon face’, retention of Na+ and water with consequent edema and hypertension etc. In addition, plasma levels of DHEA, androstenedione, and peripheral testosterone and dihydrotestosterone are also increased.
In women this-causes hirsutism, acne and amenorrhea. In men with Cushing’s disease, Cortisol suppression of LH secretion decreases testosterone production by testis, resulting in decreased libido and impotence. The increased adrenal secretion of androgen is not sufficient to compensate the decreased gonadal testosterone synthesis.
2. Ectopic ACTH syndrome:
In this case, ACTH and Cortisol hyper-secretion is randomly episodic, and the levels are often highly elevated. With a few exceptions, secretion of ACTH and Cortisol is non-suppressible with pharmacologic doses of glucocorticoid.
Typical features of Cushing’s disease are absent presumably because of rapid onset of hypercortisolism, anorexia and other manifestations of the associated malignant disease. However, hypertension and hypokalemia are frequently found due to DOC and the mineralocorticoid effects of Cortisol. Hyper- pigmentation is common in ectopic ACTH syndrome.
3. Adrenal tumors:
Adrenal adenomas causing Causing’s syndrome typically present manifestations of glucocorticoid excess, since they secrete only Cortisol. Adrenal carcinomas, on the other hand, frequently hyper-secrete multiple cortical steroids including Cortisol, androgens, DOC, aldosterone and estrogens. In this case, manifestations of hypercortisolism are usually severe and rapidly progressive.
In women, features of androgen excess are prominent; virilism may occasionally occur. Hypertension and hypokalemia are frequent and most commonly result from the mineralocorticoid effects of Cortisol; less frequently, DOC and aldosterone hyper-secretion also contribute.
Some other adrenocortical dysfunctions:
Hirsutism and virilism:
The adrenal secretory products like DHEA, DHEA sulfate and androstenedione are weak androgens. However, their peripheral conversion to testosterone and dihydrotestosterone may result in a state of androgen excess leading to hirsutism and virilism. Excessive androgen production is seen in both adrenal and ovarian disorders.
The presence of increasing number of terminal hair (which are normally coarse and pigmented and found only on scalp and eyebrows before puberty and in axillary and pubic regions at puberty) on the face, chest, back, lower abdomen and inner thighs is referred to as hirsutism.
In children, androgen excess is usually due to congenital adrenal hyperplasia or adrenal carcinoma. In women, hirsutism accompanied by amenorrhea, infertility, ovarian enlargement, and elevated plasma LH levels is typical of the polycystic ovary syndrome, whereas, Cushing’s syndrome hirsutism is accompanied by features of Cortisol excees.
In rare cases, abnormal androgen production in women increases to levels normally found only in men. Such high circulating androgen produces several somatic changes collectively referred to as virilization.
Such changes include frontal balding, deepening of voice, breast atrophy, clitoral enlargement, increased muscle mass and loss of normal female body contours. Virilism and severe androgen excess in adult women are usually due to androgen secreting adrenal or ovarian tumors. Virilism is rare in Cushing’s disease.
A congenital defect in lip hydroxylase activity causes diminished Cortisol secretion leading to excessive ACTH secretion and consequent cortical hyperplasia. The subsequent excessive production of Cortisol precursors leads to an increased availability of substrates for androgen production, causing excessive virilization both in male and female. In male, virilism may result in precocious puberty.