The following points highlight the three main categories of peptide hormones produced by anterior pituitary. The categories are:- 1. Pro-Opiomelanocortin Hormone Family 2. Somatomammotropins 3. Glycoprotein Hormones.
Category # 1. Pro-Opiomelanocortin Hormone Family:
Hormones of this family include:
(a) Corticotropin or adrenocorticotrophic hormone (ACTH) of the pars distalis,
(b) Melanocyte stimulating hormone (MSH) of pars intermedia,
ADVERTISEMENTS:
(c) Lipotropin (LPH), and
(d) Opiate peptides (endorphins).
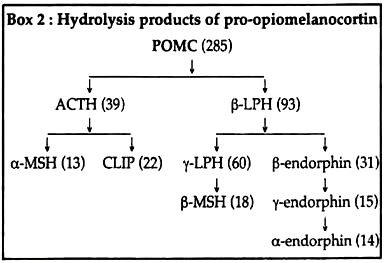
Within the corticotrophs of pars distalis, a large precursor molecule, pro-opiomelanocortin (POMC) is hydrolysed by trypsin-like enzymes into corticotropin, β-lipotropin, α-and β-melanocyte stimulating hormones (α MSH and (3 MSH), β-endorphin and an inactive 16-kD peptide.
(a) Corticotropin:
ADVERTISEMENTS:
Corticotropin is a 4.5 kD monomeric simple protein of 39 amino acid residues. The first 13 N-terminal amino acid sequences and the last 22 C-terminal residues of ACTH are, respectively, identical with the amino acid sequences of a MSH and the corticotropin-like intermediate lobe peptide (CLIP) into which ACTH may be cleaved in the intermediate lobe.
Actually these fragments are not secreted as separate hormones in human where intermediate lobe is very reduced.
In the pars intermedia (except adult human), the POMC molecule is cleaved into ACTH and a 93-amino acid peptide called P-lipotropin (β-LPH). Tryptic hydrolysis of these products next release α MSH and CLIP from, respectively, the N-terminal and C-terminal segments of ACTH molecule.
y-LPH and β-endorphin are released respectively from the corresponding segments of β-LPH. Further hydrolysis of y-LPH produces β-MSH from its C-terminal segment. Hydrolysis of β-endorphin may produce y- and α-endorphins, successively from its N-terminal segment (Box 2).
In human, β and y-LPHs are mostly released as such in the blood where they account for any MSH-like action.
Functions of ACTH:
(1) Corticotropin or ACTH binds to receptors on plasma membrane of zona fasciculata cells in adrenal cortex and enhances their proliferation to cause the growth of adrenal cortex—this is the corticotrophic effect.
(2) The hormone also stimulates the synthesis and secretion of glucocorticoid by fasciculata cells (steroidogenic effect). This steroidogenic effect is produced by several ways. ACTH enhances transfer of cholesterol from plasma LDL into fasciculata cells by increasing the number of LDL receptors on the plasma membrane.
It may activate cholesterol esterase which hydrolyses cholesterol esters into free cholesterol, thus making cholesterol available for steroidogenesis in the fasciculata cells. Corticotropin also stimulates cytochrome P450 and other enzymes that participate in steroidogenesis in the fasciculata cells.
(3) Corticotropin may act directly on adipocytes and can enhance adipose tissue lipolysis to increase the mobilisation of fatty acids from the adipose tissue into the blood, (adipokinetic effect). This effect is mediated by increased ACTH-sensitive triacylglycerol lipase activity which hydrolyses the stored triacylglycerol into free fatty acids.
Regulation of ACTH secretion:
Corticotropin shows diurnal rhythm in its secretion, being peak and nadir, normally in the morning and at midnight, respectively. Hypothalamus and corticosteroid hormones regulate its secretion from the pituitary.
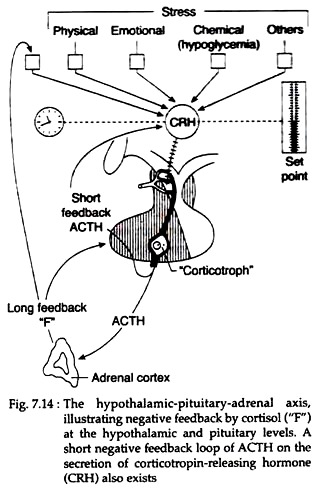
(1) Corticotropin releasing hormone (CRH) from the hypothalamus stimulates the secretion of POMC and ACTH from the pituitary. Diurnal rhythmicity stimulates the “intrinsic biological clock” located in the lymbic system of brain to secrete CRH from hypothalamus in a pulsatile manner. This CRH in turn, causes concordant diurnal secretion of ACTH. This episodic release of ACTH is independent of circulating Cortisol levels.
ADVERTISEMENTS:
(2) Negative feedback control of Cortisol on ACTH secretion occurs at both hypothalamic and pituitary levels via “fast feedback” and “slow feedback”. The first mechanism occurs too rapidly by the influence of corticosteroids on nuclear transcription of specific mRNA responsible for ACTH.
The slow feedback occurs later, by a nuclear-mediated mechanism and subsequent decrease in synthesis of ACTH. In addition to the negative feedback of corticoids, ACTH also inhibits its own secretion through short-loop feedback (Fig. 7.14).
(3) Many physical, emotional and chemical stresses (pain, trauma, hypoxia, hypoglycemia, cold, surgery, depression etc.) may stimulate ACTH secretion via vasopressin as well as CRH. Stress stimulated secretion of ACTH may often supersede the normal diurnal rhythmicity.
(b) Melanocyte-stimulating hormone/ Intermedin/MSH:
In many non-human vertebrates like elasmobranchs, lampreys, eels, catfishes and amphibians, α- and β- MSHs are secreted into blood by tire intermediate lobe and cause darkening of skin by stimulating the dispersal of melanin granules from the cell bodies of melanocytes to their peripheral processes.
MSH can also stimulate (transiently) melanin synthesis in these cells. Further, MSH also causes darkening of amphibian skin by concentrating the pigments in the iridophores.
Many other physiological roles have been suggested for MSH. This hormone is known to affect central nervous system activity in laboratory animals and human. These effects include arousal, increased motivation, longer attention span, memory retention, increased learning ability and erectile function.
In human, pregnancy is the only known physiological state in which MSH is detectable in plasma, the source of which may be placenta or pregnancy-induced pituitary intermediate lobe. The slight darkening of the skin, sometimes observed during pregnancy may result from enhanced release of MSH.
The increased pigmentation observed in human suffering from degeneration of the adrenal cortex (Addison’s disease) is also attributed to the release of excess MSH and ACTH by the pituitary. The MSH may act as trophic fetal adrenal stimulus.
(c) Lipotropins:
In human, β- and y-LPHs are mostly released as such in the blood where they account for any MSH-like action.
Category # 2. Somatomammotropins:
Growth hormone (GH) or somatotropin and prolactin (PRL) belong to this family. These hormones have 35% amino acid sequence homology with each other. GH is a 191-amino acid polypeptide hormone secreted by the somatotrophs of the anterior pituitary and prolactin is a 198-amino acid polypeptide hormone synthesised and secreted from lactotrophs of anterior pituitary.
(a) Functions of growth hormone:
This hormone promotes DNA, RNA and protein synthesis via IGF-1 (insulin-like growth factor) in liver, chondrocytes and muscles to stimulate tissue growth.
Protein anabolic effect:
Growth hormone promotes nitrogen retention and positive nitrogen balance.
Effects on carbohydrate metabolism:
GH counteracts insulin on many aspects. By decreasing glycolysis and raising blood sugar it may show diabetogenic effect. However, preceding its diabetogenic effects, it may produce transient hypoglycemia by a temporary release of insulin. It is the insulinotropic effect of GH.
GH may decrease insulin-sensitivity and hypoglycemic activity of insulin, thus exhibiting anti-insulin effects. In liver, this hormone may increase glycogen synthesis, and gluconeogenesis. It also raises muscle glycogen by decreasing glycolysis. These are glycostatic effects of GH.
Lipolytic action:
GH enhances adipose tissue lipolysis to mobilise fatty acids from adipose tissues to liver through blood. It thus increases free fatty acid level in plasma; lipid level in liver; β-oxidation and ketosis in liver.
Effects on bone and cartilage:
GH promotes the growth of bone and cartilage via insulin-like growth factor (IGF-1 or somatomedin). GH in association with IGF-1
(i) Promote the retention of Ca2+ and PO43- in the body,
(ii) Enhance the incorporation of proline in collagen, leucine into glycosoaminoglycans, SO42- into chondroitin sulfate, thymidine and uridine into nucleic acids of chondrocytes and fibroblasts,
(iii) They also increase DNA, RNA and protein synthesis in chondrocytes, their proliferation and growth. All these actions promote the growth of epiphysial cartilage to elongate long bones in infants and children, acral bone growth in facial, cranial and limb bones in adult.
Mammotrophic effect:
Due to some structural homology with prolactin, GH can bind to prolactin receptors on mammary gland and thereby stimulates some lobulo-alveolar growth and lactogenesis in mammary gland.
Effects on mineral metabolism:
GH promotes Ca2+ and PO43- retention resulting in positive Ca2+ balance which is helpful for ossification and bone growth. The hormone also shows anabolic effects on Mg2+, Na+ and K+ metabolism.
Regulation of GH secretion:
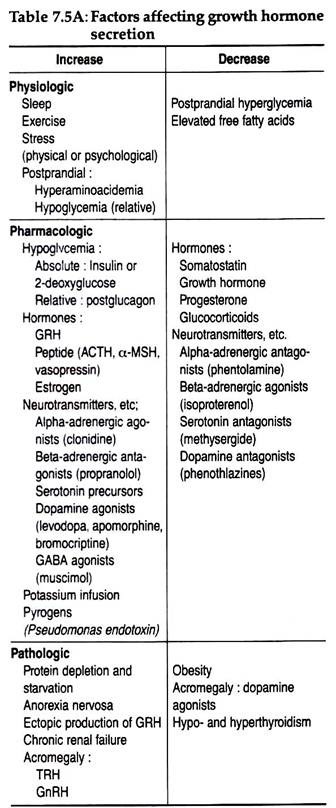
The physiologic, pathologic and pharmacologic factors influencing GH release are tabulated in Table 7.5A.
(b) Functions of prolactin:
Mammotropic or lactogenic effects:
During pregnancy, PRL enhances specific protein synthesis leading to lobulo-alveolar growth in mammary gland. It also enhances synthesis of milk proteins, like lactalbumin and casein leading to post-parturation milk secretion.
Anti-gonadotropic action:
In ovary and testis, PRL binds to receptors and decreases the steroidogenic actions of gonadotropins on those organs. It also decreases GnRH release from hypothalamus.
In vertebrates, other than human, prolactin shows many special activities, some of which are:
(i) Luteotrophic effect:
Prolactin enlarges and lengthens the secretory life of corpus luteum in pregnant rodents.
(ii) Cropsac effect:
The hormone helps in proliferation and desquamation of the crop epithelium to produce crop milk for the feeding of young birds in pigeon and dove.
(iii) Osmoregulation:
PRL controls the osmoregulation in fishes migrating between saline and fresh water.
(iv) Nesting and Brooding:
In birds, PRL stimulates these behaviours.
(v) Migration to water:
In some newts, prolactin is responsible for the water drive from terrestrial habitat when the young newt become sexually mature.
Regulation of prolactin secretion:
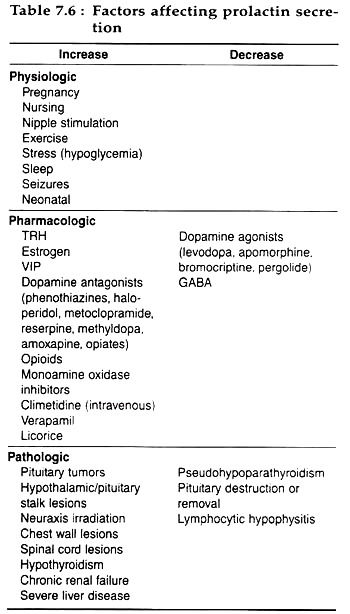
Table 7.6 summarizes the factors affecting prolactin secretion:
Category # 3. Glycoprotein Hormones:
Three adenohypophyseal hormones are glycoprotein in nature: follicle-stimulating hormone (FSH), lutenizing hormone (LH) or interstitial cell stimulating hormone (ICSH) and thyrotropin or thyroid stimulating hormone (TSH).
All these hormones contain covalently linked carbohydrate moieties at one or more positions within their structures. FSH and LH are produced by the gonadotroph cells and TSH is secreted by the thyrotroph cells of anterior pituitary.
Biochemistry and Biosynthesis:
Each of these pituitary glycoproteins is composed of two chains, α and β subunits. Within a species, the α subunit of three glycoproteins are identical to each other, but their N-linked oligosaccharide chains are generally different.
This subunit is a polypeptide of 92 amino acids. The β subunit of each hormone is structurally distinct within and between species. This subunit in human is composed of 12 residues in LHs, 112 residues in TSHs, 118 residues in FSHs.
The α and β subunits are coded by separate genes and translated separately as pre-pro-hormones, which undergo post-translational modifications as well as glycosylation to give rise to the α and β subunits, which are then non-covalently bound to form the particular hormone.
The β chain is actually responsible for specific biological action of the hormone whereas recognition and consequent binding to receptors require both subunits.
Functions of Thyrotropin:
Thyrotropin controls the function and growth of thyroid glands:
1. All steps of thyroid hormone synthesis including iodide uptake, iodination of tyrosine, coupling of iodotyrosines into T3 and T4 are stimulated by TSH. TSH shows its stimulating effects within very short time, and even when secreted in very little amount.
2. Release of stored thyroid hormones from the follicle is also controlled by TSH. Following TSH secretion, thyroid epithelium shows endocytotic uptake of colloid, proteolysis of thyroglobulin and increased activity of iodotyrosine deiodinase. All such events enhance T3 and T4 concentrations in the surrounding blood.
3. TSH secretion for prolonged time is known to enhance DNA replication, RNA transcription, protein synthesis and mitosis in thyroid follicular cells resulting in increased growth of thyroid gland.
Regulation of TSH secretion:
(a) Secretion of TSH from pituitary is regulated primarily by thyroid releasing hormone (TRH) of the hypothalamus. In general, TRH enhances the synthesis of TSH by stimulating transcription of thyrotropin gene in thyrotrophs.
The hormone also stimulates phospholipase C-polyphosphoinositol system in the thyrotrophs to increase intracellular IP3 and Ca2+ which act as second messengers to stimulate the release of thyrotropin (TSH).
(b) Serum level of thyroid hormones if rises, then it can decrease TSH secretion from pituitary by inhibiting the secretion of TRH from hypothalamus through long-loop feedback inhibition,
(c) Somatostatin released from hypothalamus may decrease TSH release by counteracting hypothalamic TRH.
Functions of FSH:
(1) In females, FSH promotes proliferation of granulosa cells to cause ovarian follicular growth. It also upregulates the LH receptors on ovarian granulosa cells which augments the LH action on ovarian follicle. Such augmented activity of LH causes ovulation as well as post-ovulatory progesterone secretion.
(2) FSH also stimulates ovulation by increasing the synthesis and release of a serine protease, plasminogen-activator which acts as the ovulation-initiator.
(3) In males, FSH stimulates the growth of seminiferous tubules and spermatogenesis. This function is mediated by the proteins synthesised from Sertoli cells upon FSH stimulation that carry testosterone from the Leydig cells to spermatogenic cells.
Function of LH:
(1) In women near the end of follicular phase of menstrual cycle, a rise in LH secretion causes the synthesis and release of plasminogen activator by granulosa cells. This protease then hydrolyses inactive plasminogen into active plasmin which initiates the degradation of follicular wall leading to ovulation.
(2) Following ovulation, the continued action of LH on follicular granulosa cells causes their proliferation and changes the ruptured follicle into cropus luteum.
(3) LH also stimulates ovarian steroidogenesis in luteal cells to secrete progesterone.
(4) In males, it stimulates testosterone synthesis from Leydig cells.
(5) Indirectly, LH stimulates spermatogenesis by way of its effect on testosterone biosynthesis, which is required for maturation of germ cells.
(6) It also helps in the development of secondary sexual characters in male.
Regulation of FSH and LH secretion:
Pituitary secretion of FSH and LH is controlled by gonadotropin-releasing hormone (GnRH) from hypothalamus:
(a) Episodic secretion:
In both males and females, secretion of LH and FSH is episodic which is mediated by a concordant episodic release of GnRH. This pulsatile nature of GnRH release is critical for sustaining gonadotropin secretion. A continuous, prolonged infusion of GnRH in women evokes an initial increase in LH and FSH followed by prolonged suppression of gonadotropin secretion. This is due to down regulation of GnRH receptors on pituitary gonadotrophs.
(b) Positive feedback:
Circulating sex steroids control GnRH secretion and thus LH and FSH secretion. During menstrual cycle estrogens provide a positive influence on GnRH effects on LH and FSH secretion. The rise in estrogen during the follicular phase is the stimulus for LH and FSH ovulatory surge. Progesterone amplifies the duration of LH and FSH surge and augments the effects of estrogen.
After this mid-cycle surge, ovulation takes place about 10-12 hours after the LH peak and 24-36 hours after estrogen peak. The remaining cells of the ruptured follicle then under the influence of LH are converted to a progesterone secreting corpus luteum. After about 12 days, the corpus luteum involutes, resulting in decreased estrogen and progesterone levels.
(c) Negative feedback:
The decline in serum gonadotropins in the luteal phase and in serum FSH in the late follicular phase of menstrual cycle result from the negative feedback effects of progesterone-estrogen and estrogen, respectively.
Primary gonadal failure or menopause in women is characterised by elevated LH and FSH, which can be suppressed by long-term, high-dose estrogen therapy. In males, inhibin, a polypeptide secreted by the Sertoli cells of seminiferous tubules, inhibits FSH secretion by negative feedback.
(d) Gonadotropin secretion is also modulated by neurogenic factors like photo-period, visual, olfactory, emotional and sexual stimuli.