In this article we will discuss about:- 1. Biochemistry of Thyroid Hormone 2. Synthesis of Thyroid Hormone 3. Secretion.
Biochemistry of Thyroid Hormone:
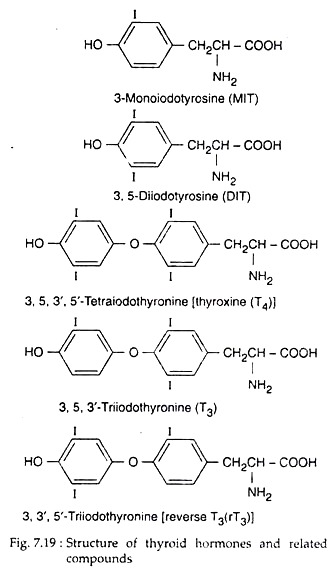
Thyroid follicular cells synthesise and secrete two iodinated amino acids as hormones, viz., thyroxine or T4 or 3, 5, 3′, 5′-tetraiodothyronine and T3 or 3, 5, 3′- triiodothyronine (Fig. 7.19). Instead of these hormones, the C cells of mammalian thyroid secrete a polypeptide hormone calcitonin.
Synthesis of Thyroid Hormone:
The overall synthesis process of thyroid hormones (T3 and T4) involves the following major steps:
ADVERTISEMENTS:
1. Iodide trap:
Thyroid follicular cells actively collect inorganic iodides (I−) from the circulation against steep electrochemical gradients with the help of carrier protein, sodium iodide symporter located in the basal plasma membrane in association with Na+K+ ATPase. The later provides energy for functioning of iodide symporter which collects about ~33% of the body I− in the thyroid and concentrate that I− by 25-50 times the serum I– concentration.
Such iodide uptake into the thyroid follicle is an example of secondary active transport, sometimes improperly referred to as “iodide pump”. TSH stimulates the iodide transport and this transport is inhibited by some anions, notably perchlorate and thiocyanate.
2. Oxidation of iodide:
ADVERTISEMENTS:
A tetrameric heme enzyme, thyroperoxidase of the apical plasma membrane of follicle cells then binds I–. The enzyme bound I– is then oxidised to active iodine by H2O2, generated by an NADPH dependent enzyme system in presence of Ca2+. The active iodine may be iodinium ion (I+), hypo-iodate (IO−) or free iodine radical (I).
3. lodination of tyrosyl in thyroglobulin:
The thyroperoxidase enzyme then incorporates active iodine into C3 and C5 of some tyrosyl groups of thyroglobulin (TG) and form monoiodotyrosine (MIT) and diiodotyrosine (DIT) residues, respectively.
4. Coupling of iodotyrosines in thyroglobulin:
ADVERTISEMENTS:
The coupling of iodo-tyrosyl residues in thyroglobulin is again catalysed by thyroperoxidase. It is probably an intra-molecular process that involves:
(i) Oxidation of iodo-tyrosine to an active form by thyroperoxidase,
(ii) Coupling of activated iodo-tyrosyl residues within the same thyroglobulin molecule to form a quinol ether intermediate and
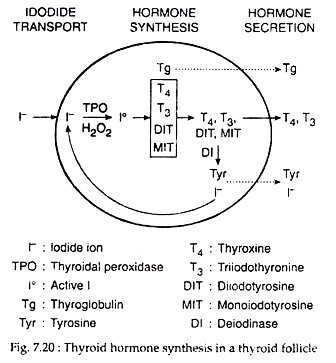
(iii) Spliting of the quinol ether to form iodothyronine with conversion of the alanine side chain of the donor iodo-tyrosine to dehydroalanine (Fig. 7.20). Dimeric structure of thyroglobulin actually helps this process. Within it, two molecules of DIT may couple to form T4, and an MIT and a DIT molecule may couple to form T3.
Thyroglobulin:
The main intra-thyroidal storage form of thyroid hormone is thyroglobulin which is a large glycoprotein molecule containing 5,496 amino acids, with a molecular weight of about 660,000. In thyroglobulin containing 0.5% iodine, there would be 5 molecules of MIT, 4.5 molecules of DIT, 2.5 molecules of thyroxine (T4) and 0.7 molecules of triiodothyronine (T3).
There are four tyrosyl sites for hormonogenesis on the thyroglobulin molecule; one site is located at the amino-terminal end of the molecule, and the other three are located in a sequence of 600 amino acids at the carboxyl terminal end.
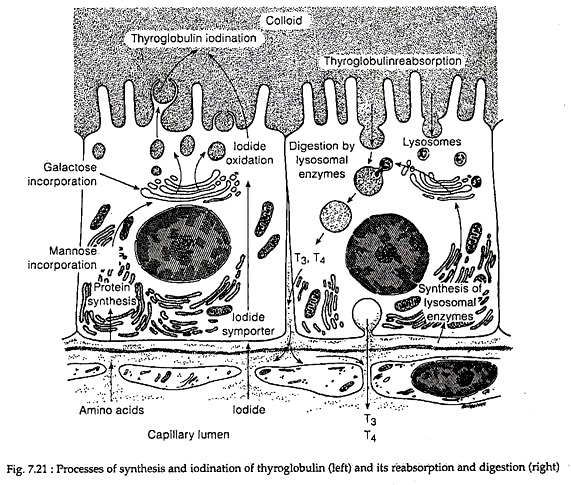
In the Golgi apparatus of follicle cells, the thyroglobulin dimers are incorporated into exocytotic vesicles that fuse with the basement membrane and release the thyroglobulin into the follicular lumen. There, at the apical colloid border, thyroglobulin is iodinated and are stored in colloid (Fig. 7.21).
Storage, Release and Transport:
The thyroid hormones (T4 and T3) formed within the thyroglobulin molecules at the apical colloid border remain stored in the colloid. During release, at the cell-colloid interface, colloid is engulfed into colloid vesicles by the process of macro-pinocytosis or micro-pinocytosis and is absorbed into the thyroid cell.
The cellular lysosome containing proteases, endo-peptidases, glycoside hydrolyase, then fuse with colloid vesicles. In the fused vesicles, hydrolysis of thyroglobulin occurs, releasing T4, T3, DIT MIT, peptide fragments and amino acids.
T3 and T4 pass into blood across the basal cell membrane. DIT and MIT are de-iodinated by NADPH dependent microsomal deiodinase and the released iodine is recycled into tyrosyl iodination. Thyroid hormone secretion is stimulated by TSH. Thyroglobulin proteolysis is inhibited by excess iodide and by lithium.
In blood, little amount of T4 and T3 are carried as free T4 and T3, while the maximum amount of both hormones are carried as bound form with thyroid binding globulin (TBG). Some amount of T4 also remains bound to thyroxine binding pre-albumin (TBPA) and serum albumin.
Secretion of Thyroid Hormone:
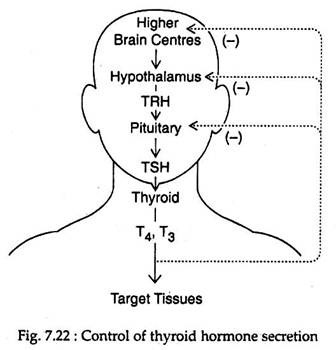
The secretion of thyroid hormone is regulated by thyroid stimulating hormone (TSH) from the pituitary gland that in turn is regulated by thyrotropin releasing hormone (TRH) of hypothalamus. Thyroid hormones feed back at the pituitary and hypothalamic levels to inhibit TSH secretion (Fig. 7.22).
TSH secretion is inhibited by stress in a number of species. Recent experimental evidences suggest that acute neuroendocrine reflex exists for TSH as well as TRH release.
High level of plasma T4 exerts .negative feedback action on pituitary TSH production. T4 also controls TRH synthesis by regulating gene expression of the TRH pro-hormone in the thyrotrophic area of the hypothalamus.