In this article we will discuss about:- 1. Meaning of Complement System 2. Components of Complement System 3. Functions.
Meaning of Complement System:
Humoral immunity is mediated by secreted antibodies, and its physiological function is defence against extracellular microbes and microbial toxins. The antibody mediated elimination of antigens always requires the participation of some other effector systems like phagocytes and complement proteins.
The complement system is a group of serum and cell surface proteins and glycoproteins that interact with each other and with other molecules of immune system in a highly regulated manner to provide many effector functions of humoral immunity. It is also an important effector mechanism of innate immunity.
The name of complement system was derived from the experiment performed by Jules Bordet (1890s) shortly after the discovery of antibodies. He demonstrated that fresh serum containing antibacterial antibody if interacted with bacteria at 37°C, the bacteria were lysed.
ADVERTISEMENTS:
If, however, the serum was heated to 56°C or more, it losts its lytic capacity. From this experiment he concluded that serum must contain some heat-labile component that assists or complements the lytic function of antibodies and this component was later given the name complement by P. Ehrlich.
Site of Synthesis:
The complement system is composed of many proteins and glycoproteins which are synthesised primarily by liver hepatocytes, blood monocytes, and tissue macrophages, epithelial cells of gastrointestinal and urinogenital tracts.
Components of Complement System:
The components of complement system constitute 5% of serum globulin fraction (by weight). Most of them are pro-enzymes or zymogens which circulate in serum as functionally inactive form.
ADVERTISEMENTS:
Complement components are designated by numerals (C1-C9) by letter symbols (e.g., Factor D) or by trivial names (e.g., homologous restriction factor). The peptide fragments formed by activation of a component are designated by small letters.
In most cases, the smallest fragment resulting from cleavage of a component is designated ‘a’ and the larger fragment is designated as ‘b’ (2a is the exception). The complexes having an enzymatic activity are designated by a bar over the number of symbol e.g., C4b2a, C3bBb.
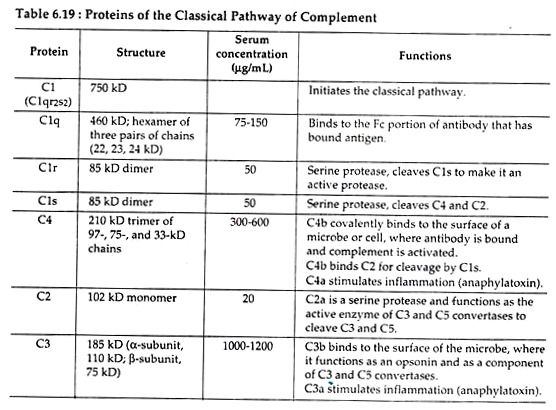
Of different serum proteins C1, C2, C3, C4 and C5 are the early step-components with enzymatic function, whereas C6-C9 are non-enzymatic and referred to as late-step components.
Activation of Complement:
The complement system becomes activated or show their response to microbes by means of a series of proteolytic cleavage or proteolytic cascade which removes an inhibitory fragment and exposes the active site.
ADVERTISEMENTS:
Each enzyme molecule activated at one step, can generate multiple activated enzyme molecules at the next step (cascade activity). However, many of the activated components become inactivated shortly if they do not react with the next component in the cascade sequence.
There are three major pathways of complement activation; each one is initiated by specific set of stimuli.
These are:
(1) Classical pathway
(2) Alternative pathway, and
(3) Lectin pathway.
The names classical and alternative arose because the classical pathway was discovered and characterised first, but the alternative pathway is phylor genetically older.
Although the pathways of complement activation differ in how they are initiated, all of them result in the generation of enzyme complexes that are able to cleave the most abundant complement protein, C3. Complement activation consists of early steps which result in the proteolysis of C3 and late steps, which lead to the formation of a protein complex that lyses cells.
In the early steps, two proteolytic products of C3 called C3a and C3b are generated. C3b then becomes covalently attached to the microbial cell surface or to the antibody molecules where complement is activated, and here it binds to other complement proteins and initiates the late steps of complement activation. The pathways differ in how C3b is produced, that is, in the early steps, but they share the same late steps.
ADVERTISEMENTS:
The detailed descriptions of different pathways are:
1. Classical pathway:
This pathway of complement activation is initiated by the binding of complement proteins to antigen-antibody complexes or with the binding of antibody to antigen on a suitable target, such as bacterial cell. This pathway helps in humoral immunity.
Activating molecules:
Antigen complexed with IgM and certain classes of IgG molecules (e.g., human IgGl, IgG2 and IgG3) can initiate the classical pathway. However, some viral membrane components, C-reactive protein, bacterial lipopolysaccharide and trypsin-like protease can also activate the CI component of classical system.
Components of classical pathway:
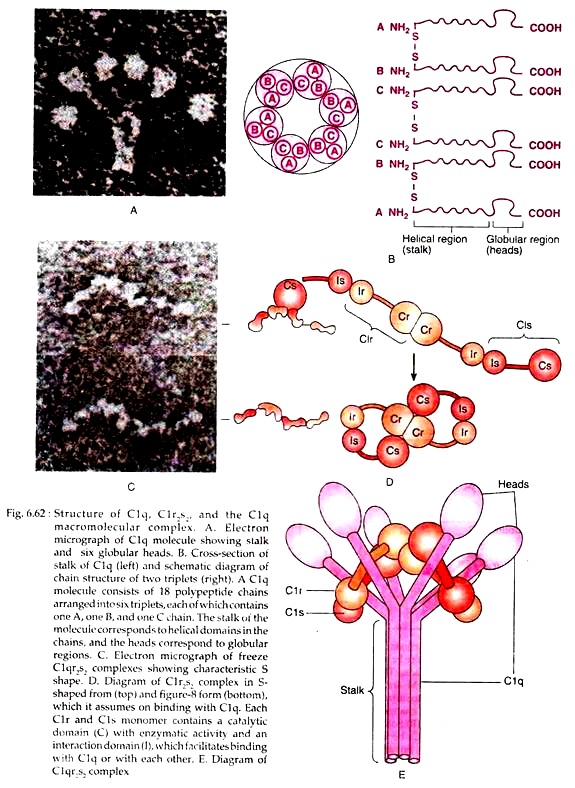
The main components of classical pathway include C1, C2, C3 and C4, all of which remain in inactive form (Table 6.19). C1 in serum is a macromecular complex consisting of C1q and two molecules each of C1r and C1s, held together in a complex (C1r2s2) stabilized by Ca2+ ions.
The C1q molecule is composed of 18 polypeptides that associate to form six collagen-like triple helical arms, the tips of which bind to exposed C1q-binding sites in the CH2 domain of antibody molecule (Fig. 6.62(b). The C1r2S2 complex can exist in two configurations. When it is free and not bound to C1q, it assumes an S-shaped form; on binding to C1q, C1qr2s2 assumes a 8-like configuration (Fig. 6.62).
The C4 component is a glycoprotein containing three polypeptides α, β and y and it has two isomers C4a and C4b. Native C3 components consist of two polypeptide chains, α and β and it also exists in two forms C3a and C3b.
Activation:
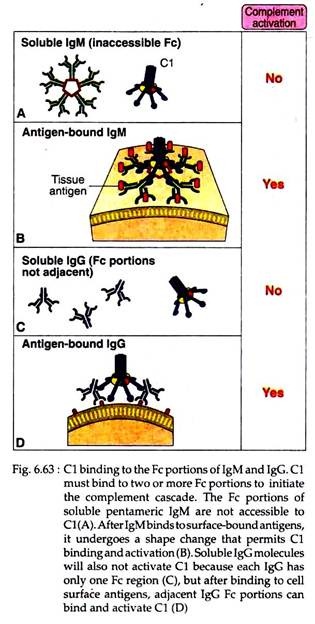
The classical pathway is activated when the first component-C1 binds with CH2 domains of IgG or CH3 domain of IgM molecules that have bound antigen. It should be noted here that single C1q molecule must bind simultaneously to at least two Fc portions of Ig. Each IgFc region has a single C1q-binding site, and each C1q molecule must bind to two Ig heavy chains to be activated.
This requirement explains why antibodies bound to antigens and not free circulating antibodies can-initiate classical pathway activation (Fig. 6.63). As each IgG molecule has only one Fc region, multiple IgG molecules must be brought together only when they bind to a multivalent antigen. Free IgM is pentameric, but it cannot bind C1q, because Fc regions of free IgM are inaccessible to C1q.
Binding of an antigen to IgM induces a conformational change that exposes the C1q binding sites in the Fc regions of IgM and allows C1q to bind. However, because of its pentameric structure one IgM molecule can bind two C1q molecules and that is why IgM is more efficient complement binding antibody than IgG.
The binding of C1q to IgG or IgM brings conformational changes in C1r molecules that converts C1r to active C![]() , C
, C![]() then converts C1s to active C
then converts C1s to active C![]() which has two substrates-C4 and C2.
which has two substrates-C4 and C2.
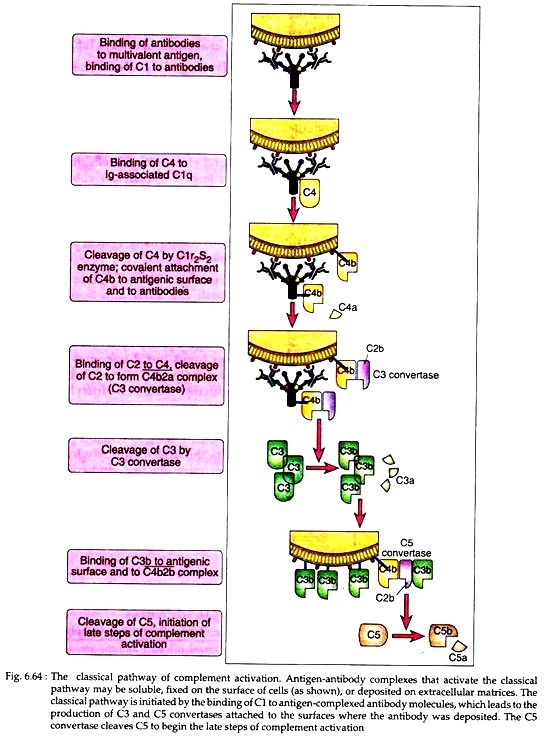
The C4 containing three polypeptide chains α, β, and y is activated when C1s hydrolyses a small fragment (C4a) from the amino terminus of the α chain. This hydrolysis exposes a binding site on the larger fragment (C4b). The C4b then attaches to the target surface in the vicinity of C1 and the C2 pro-enzyme then attaches to the exposed binding site on C4b, where C2 is then cleaved by the neighbouring C![]() .
.
The resulting C![]() complex is called C3 convertase as it can convert pro-enzyme C3 into enzymatically active form. The smaller fragment released after C4 cleavage, C4a acts as anaphylatoxin or mediator of inflammation is du-fused away and do not participate directly in the complement cascade.
complex is called C3 convertase as it can convert pro-enzyme C3 into enzymatically active form. The smaller fragment released after C4 cleavage, C4a acts as anaphylatoxin or mediator of inflammation is du-fused away and do not participate directly in the complement cascade.
C4 is homologous to C3. C4b has an internal thioester bond, as in C3b, that forms covalent amide or ester linkage with the antigen-antibody complex or with the adjacent surface of a cell to which the antibody is bound. This attachment ensures that classical pathway activation proceeds on a cell surface or immune complex.
In the next step, the C3 convertase hydrolyses a small fragment (C3a) from the amino terminus of the α chain of C3 generating C3b. A single C3 convertase can generate over 200 molecules of C3b, thus causing tremendous amplification at this step of enzymatic interaction. Some C3b then binds to C![]() to form tri-molecular complex C
to form tri-molecular complex C![]() , called C5 convertase (Fig. 6.64).
, called C5 convertase (Fig. 6.64).
The C3b component of this complex actually causes some conformational changes in the C5, so that the C![]() component can cleave C5 into C5a and C5b. Of these C5a diffuses away, and C5b attaches to next substrate C6. Formation of C5 convertase initiates the late step of complement activation which is discussed later.
component can cleave C5 into C5a and C5b. Of these C5a diffuses away, and C5b attaches to next substrate C6. Formation of C5 convertase initiates the late step of complement activation which is discussed later.
2. Alternate pathway:
This pathway is initiated on microbial surfaces without the requirement for specific immune responses. It is an antibody independent pathway and an important mechanism of innate immunity against infectious organisms.
Components of alternate pathway:
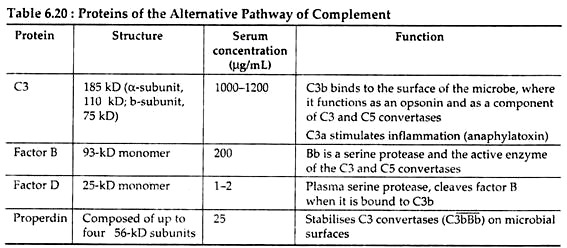
Serum proteins C3, factor B, factor D and properdin are the main components participating in alternate pathway (Table 6.20).
Activating molecules:
The alternate pathway is antibody independent. Many of Gram negative and Gram positive bacteria, lipopolysaccharides from bacterial cell wall, teichoic acid from Gram positive cell wall, zymosan of fungal and yeast cell wall, some viruses and virus-infected cells, tumor cells, parasites (e.g., Trypanosoma), cobra venom, heterogeneous erythrocytes, human IgG, IgA, IgE in complexes, pure carbohydrate (insulin, agarose) etc. can initiate or activate alternate pathway of complement system and help in innate immunity.
Activation:
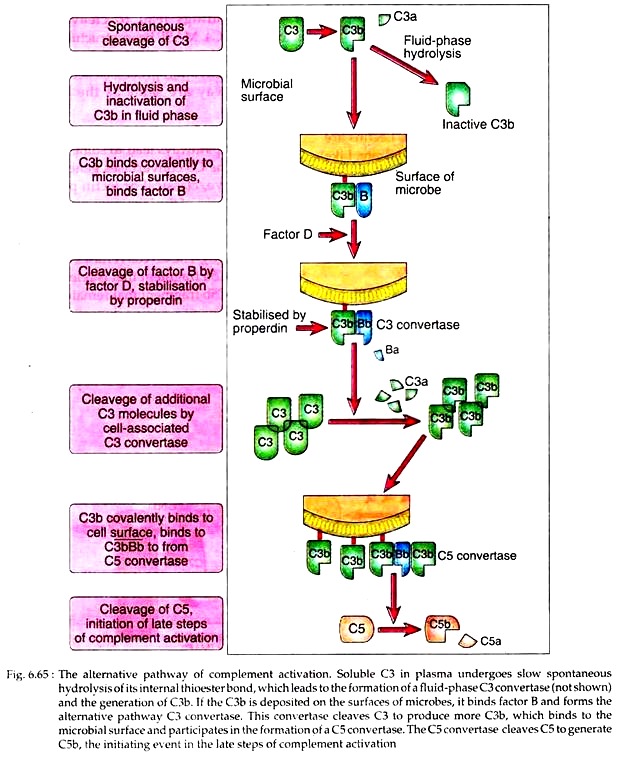
The alternate pathway starts with the slow and spontaneous hydrolysis of C3 to yield C3a and C3b. This C3b can bind to foreign surface antigens or even to host’s own cells. The internal thioester bond in C3 becomes unstable when the molecules are cleaved, and it reacts with the amino or hydroxyl groups of cell surface proteins or polysaccharides to form amide or ester bonds. If these bonds are not formed, the C3b remains in the fluid phase, its thioester is quickly hydrolysed and it becomes inactive, and complement activation is stopped.
The bound C3b then binds a plasma protein, called factor B that acts as the substrate for factor D. Factor D then hydrolases C3b bound factor B, releasing small component Ba and generates C![]() . This C
. This C![]() has C3 convertase activity and thus is analogous to C
has C3 convertase activity and thus is analogous to C![]() . complex of classical pathway. This complex has a half-life of only 5 minutes, unless the serum protein properdin binds to it, which stabilizes it and also extends its half-life to 30 minutes.
. complex of classical pathway. This complex has a half-life of only 5 minutes, unless the serum protein properdin binds to it, which stabilizes it and also extends its half-life to 30 minutes.
The C![]() , in the next step activates un-hydrolysed C3 to generate more 3Cb. As a result, more than 2 × 106 molecules of C3b can be deposited on antigen surface within a few minutes. In this way, C
, in the next step activates un-hydrolysed C3 to generate more 3Cb. As a result, more than 2 × 106 molecules of C3b can be deposited on antigen surface within a few minutes. In this way, C![]() generates C
generates C![]() complex, which has C5 convertase activity, analogous to C
complex, which has C5 convertase activity, analogous to C![]() complex of classical pathway. The C5 convertase then cleaves C5 and initiates the late steps of complement activation (Fig. 6.65).
complex of classical pathway. The C5 convertase then cleaves C5 and initiates the late steps of complement activation (Fig. 6.65).
3. Lectin pathway:
Lectin pathway is a recently discovered means of complement activation. Lectins are proteins that can bind to a carbohydrate. Like alternate pathway, lectin pathway does not require antibody for its activation.
Activation of lectin pathway:
Binding of microbial polysaccharides or glycoprotein to circulating lectins, such as mannose-binding lectin (MBL) activate this pathway.
Components of lectin pathway:
MBL is a protein, produced during inflammatory responses and it acts in a similar way to that of C1q. Other components involved in lectin pathway are those that participate in classical pathway.
Activation:
Following binding of MBL to the surface of a cell or pathogen, MBL- associated serine protease, or MASP binds to it. Such bindings lead to cleavage and activation of C4. MASP bears structure similar to C1r and C1s and mimics their activities. Rest of the activation processes occurring in lectin pathway is same as the classical pathway.
Late steps of complement activation:
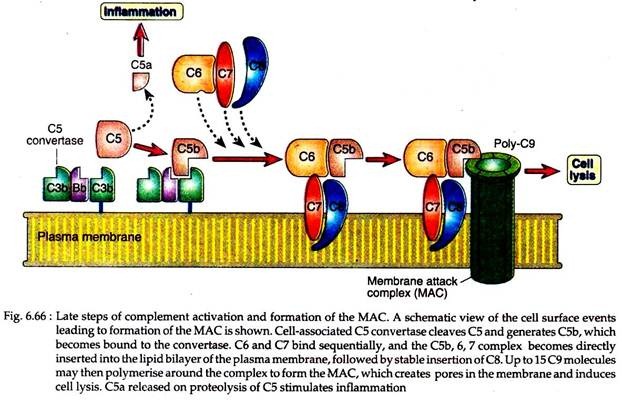
All complement pathways lead to some common late steps. The C5 convertases generated by the alternative, classical or lectin pathways initiate activation of the late components of the complement system, which culminate in the formation of the cytocidal membrane attack complex (MAC).
The C5 convertases cleave C5 into a small C5a fragment that is released and a two-chain C5b fragment that remains bound to the complement proteins deposited on the cell surface.
The remaining components of complement cascade, C6, C7, C8 and C9 are structurally related proteins without enzymatic activity. C5b then transiently bind to next proteins of cascade, C6 and C7 resulting in the formation of C5b67. The C7 component of this complex is hydrophobic and it inserts into the lipid bilayer of cell membranes, where it becomes a high affinity receptor for C8 molecule (Fig. 6.66).
The C8 protein has 3 polypeptides, one of which binds to the C5b67 complex; another inserts into lipid bilayer of the cell membrane. However, this C5b678 complex has a limited ability to lyse the cells. The formation of a fully active MAC is accomplished by the binding of C9, the final component of complement cascade to the C5b-8 complex.
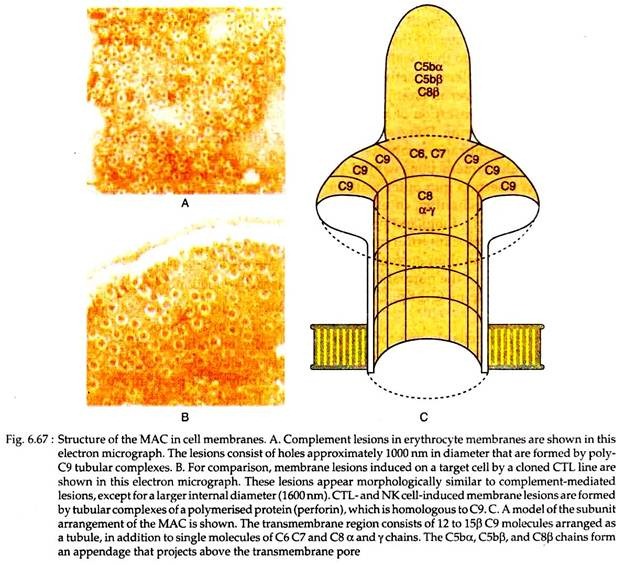
C9 is a serum protein that is structurally similar to perforin, a cytolytic granule protein found in cytolytic T cells. It polymerises at the site of the bound C5b-8 to form pores in plasma membrane (Fig. 6.67).
These pores are 1000 nm in diameter and they form channels that allow free entry of water and ions in the cell. This entry of water results in osmotic swelling and rupture of the cells on whose surface the MAC is deposited.
Functions of Complement System:
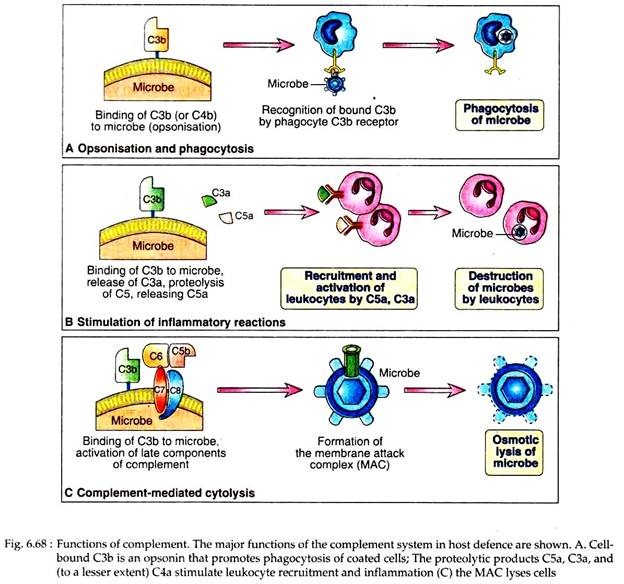
The principal effector functions of the complement system in innate and specific humoral immunity are to promote phagocytosis of microbes on which complement is activated, to stimulate inflammation and to induce lysis of these microbes. Both the MAC and the proteolytic fragments generated during activation processes are involved in mediating host’s immune responses.
Different biological activities of complement system are described below:
1. Complement-mediated Cytolysis:
Complement mediated lysis of foreign organism is performed by the MAC, generated following classical or alternative or lectin pathway. A number of microorganism, viruses, erythrocytes and nucleated cells may be lysed by MAC. Viruses derive their envelope from the infected host cells and thus are susceptible to pore formation by MAC.
Some bacteria have high cell-wall lipopolysaccharides (E. coli, Salmonella) that prevent insertion of the MAC into their wall, making them resistant to complement activity. The thick peptidoglycan layer in Gram positive bacteria also prevents insertion of the MAC into their inner membrane.
Some strains of Neisseria have membrane proteins that undergo non-covalent interactions with MAC that prevent its insertion into outer wall. In addition to this mechanism of evasion, various viruses, bacteria, fungi and protozoa contain proteins that can interrupt the complement cascade on their surfaces, thus mimicking the effects of the normal complement regulatory proteins C4bBp, CRI and DAF.
Lysis of nucleated cells requires formation of multiple MAC, whereas a single MAC can destroy one red blood cell; these nucleated cells tend to be more resistant to complement-mediated lysis than RBC. Many cancer cells can endocytosis the MAC and can repair the damage and thus restore the osmotic stability.
2. Opsonisation and Phagocytosis:
As described earlier, activation of both classical and alternative pathways leads to generation of C3b and iC3b, which act as opsonins to promote the phagocytosis of antigens coated with these peptides.
Microbes on which complement is activated by the different pathways become coated with C3b, iC3b or C4b and are phagocytosed by the binding of these proteins to specific receptors on macrophages and neutrophils (Fig. 6.68). C3b and C4b bind to CR1 and iC3b binds to CR3 (Mac-1) and CR4.
CR1 itself is inefficient in inducing the phagocytosis of C3b-coated microbes, but its ability to do so is enhanced if the microbes are coated with IgG antibodies. Macrophage activation by the cytokine IFN-y also enhances CR1-mediated phagocytosis.
C3b can enhance the number of CR1 from 5,000 on resting phagocytes to 50,000 on activated cells, thus greatly facilitating their phagocytosis of C3b- coated antigen. In innate and adaptive immunities C3b and iC3b-dependent phagocytosis of microorganisms is a major defense mechanism. Humans and mice lacking C3 protein are extremely susceptible to lethal bacterial infections.
3. Inflammatory Response:
The proteolytic complement fragments like C5a, C4a and C3a induce acute inflammation by activating mast cells and neutrophils. These peptides are called anaphylatoxins because the mast cell reactions they trigger are characteristic of anaphylaxis. These molecules bind to mast cells and cause degranulation, with the release of vasoactive mediators such as histamine.
Many cells such as neutrophils, eosinophils, basophils, monocytes, macrophages, mast cells, endothelial cells, smooth muscle cells, epithelial cells have C5a receptors which is a member of G-protein coupled receptor family. In neutrophils, C5a stimulates motility, firm adhesion to endothelial cells and at high doses, stimulation of the respiratory burst and production of reactive oxygen intermediates.
Moreover, C5a may act directly on vascular permeability and expression of P-selectin, which promotes neutrophil binding. Among different proteolytic complement fragments, C5a is the most potent mediators of mast cell degranulation, C3a is about 20-fold less potent and C4a is about 2,500-fold less.
4. Neutralisation:
The binding of serum antibody to the repeating subunits of the viral structural proteins creates particulate immune complexes ideally suited for complement activation by classical pathway. The complement system thus activated may neutralize the viral particles in a number of ways.
In vitro studies revealed that C3b component can facilitate aggregate formation in the presence of as little as two molecules of antibody per viron. The binding of antibody and/or complement to the virus-surface can create a protein coating that can neutralize viral infectivity by blocking attachment to susceptible host cells.
Complement deposits on viral particles may facilitate binding of virus to cells like phagocytic cells possessing Fc or type 1 complement receptor. As a result, phagocytosis or intracellular destruction of ingested viral particle increases. Finally, complement may be effective in lysing many enveloped viruses, resulting in fragmentation of the envelope and disintegration of the nucleocapsid.
5. Solubilisation of Immune Complexes:
By binding to antigen antibody complexes, complement proteins promote the solubilisation of these complexes and their clearance by phagocytosis. When immune complexes accumulate in the blood, they may be deposited in vessel walls, leading to inflammatory reactions that damage surrounding tissues.
Complement activation on Ig molecules can sterically block the Fc-Fc interactions that may form during immune complex formation, thereby promoting dissolution of the immune complexes. In addition, immune complexes, with attached C3b are bound to CR1 on erythrocytes and the complexes are cleared by phagocytosis in liver or spleen.
6. Initiation of Humoral Immune Response:
The C3d protein is generated when complement is activated by an antigens. Complement activation results in the covalent attachment of C3b and its cleavage product C3d to the antigen. B lymphocytes can bind the antigen through their Ig receptors and simultaneously it binds the attached C3d through CR2. This combined signals result in B cell activation.
7. Respiratory Burst in Leucocytes:
C5a, a complement proteolytic fragment may stimulate oxidative metabolism in leucocytes to produce the respiratory burst, i.e., the sudden manifold rise in O2 consumption seen in those cells during phagocytosis. It helps in the formation of superoxide anions (O-2), singlet oxygen (1O2), free hydroxyl radical (OH), which help to destroy the phagocytized microbes.
8. Chemotactic Action:
C5a and C5b67 can induce monocyte and neutrophils to adhere to vascular endothelial cells and migrate towards the site of complement activation in the tissue. In this way, complement products help in leucocyte chemo taxis towards affected tissues. Besides functioning as an effector mechanism of host defence, the complement system is also involved in several pathologic conditions.
Receptors for Complement Proteins:
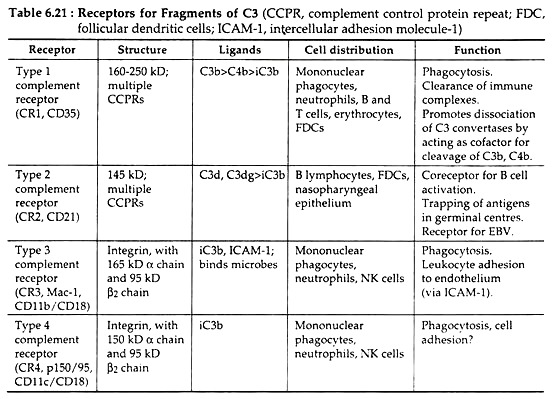
Most of the biological activities of the complement system are mediated by the binding of complement fragments to membrane receptors expressed on various cell types. The type 1 complement receptors (CRI or CD35) act mainly to promote phagocytosis of C3b- and C4b-coated particles and clearance of immune complexes from the circulation.
The type 2 receptor (CR2 or CD21) functions mainly to stimulate humoral immune responses by enhancing B cell activation by antigen and by promoting the trapping of antigen-antibody complexes in germinal centres.
The type 3 complement receptor, also known as Mac-1 (CR3, CD11b, and CD18) is an integrin that functions as a receptor for the iC3b fragment produced by proteolysis of C3b. The type 4 complement receptor (CR4) is another integrin with almost similar type of function as that of Mac-1. The different cell types expressing these receptors, their ligand, structure and specific functions are tabulated in Table 6.21.
Regulation of complement activation:
Complement activation and stability of active complement proteins are tightly regulated because of two main reasons. Firstly, low level complement activation goes on spontaneously, and if such activation is allowed to proceed on normal cells, the result can damage normal cells and tissues.
Secondly, even when complement is activated where needed, such as on microbial cells or antigen-antibody complexes, it needs to be controlled because degradation products of complement proteins may diffuse to adjacent cells and can injure them. So there are different regulatory mechanisms to prevent complement activation on normal host cells and to limit the duration of complement activation.
(i) A plasma protein called C1 inhibitor (C1 INH) may inhibit the proteolytic activity of C1r and C1s. It is a serine protease inhibitor (serpin) that mimics the normal substrates of C1r and C1s. When C1q binds to an antibody and begins the process of complement activation, C1 INH becomes a target of the enzymatic activity of the bound C1r2-C1s2.
As a result, C1 INH is cleaved by and becomes covalently attached to these complement proteins, resulting the dissociation of C1r2- C1s2 tetramer from C1q. Consequently, the activation of classical pathway is stopped. In this way, C1 INH prevents the accumulation of enzymatically active C1r2-C1s2 in the plasma and limits the time for which it is available to activate subsequent steps in the complement cascade.
(ii) Deposited C3b on the surfaces of normal mammalian cells may be bound by several membrane proteins, including membrane cofactor protein (MCP or CD46), type-1 complement receptor (CR1), decay-accelerating factor (DAF) and a plasma protein called Factor H. C4b deposited on the cell surface is similarly bound by DAF, CR1 and another plasma protein called C4-binding protein (C4BP).
Such bindings between these proteins and C3b or C4b competitively inhibit the binding of other components of the C3 convertase, such as Bb of the alternative pathway and C2a of the classical pathway. As a result, further progression of the complement cascade activation is blocked. Such regulators of complement can selectively inhibit complement activation on host cells but allow complement activation to proceed on microbes.
(iii) Cell-associated C3b is proteolytically degraded by a plasma serine protease called Factor I. MCP, factor H, C4BP and CR1 all serve as cofactor for Factor I-mediated cleavage of C3b. Thus, these regulatory host cell proteins promote proteolytic degradation of complement proteins.
The same regulatory protein also causes dissociation of C3b- containing complexes. Cleavage of C3b generates fragments called iC3b, C3d and C3dg which do not participate in complement activation but may be recognised by receptors on phagocytes.
(iv) A membrane protein called CD59 can inhibit the formation of MAC on host cells. CD59 is a glycophosphatidylinositol-linked protein, expressed on many cell types. This protein can incorporate itself into growing MACs after the membrane insertion of C5b-8, thereby inhibiting subsequent addition of C9 molecules. CD59 is not present on microbes but on normal host cells where it limits the formation of MAC.
MAC formation may also be inhibited by a plasma protein called S protein which acts by binding to soluble C5b67 complexes and thereby preventing their insertion into cell membrane near the site where the complement was initiated. Such inhibitors of MAC in the plasma and in host cell membrane ensure that lysis of innocent bystander cells does not occur near the site of complement activation.
In normal cells, the regulatory proteins prevent complement activation. However, in a number of immunological diseases, complement mediated phagocytosis and damage to normal cells are important pathogenetic mechanisms.
In such cases, large amount of antibodies are deposited on host cells, generating enough active complement proteins, that the regulatory molecules are unable to control the complement activation.