Here endocrine structures in insects will be discussed according to their origin.
Endocrine Organs of Nervous Origin:
In insects, neurosecretory cells are numerous and have important functions. Recent work has revealed that there are two types of neurosecretory cells: type A which stain with para-aldehyde fuchsin, and type B which do not. All cells possess electron dense granules. The neurosecretory cells can produce blue colour from the reflected light due to the light scattering effect of colloidal-sized particles.
Protocerebrum:
It is the most complex part of the insect brain consisting of several distinct cell masses and regions of neuropile. The pars intercerebralis, part of protocerebrum is located in the dorsal median region above the proto-cerebral bridge and central body. It contains two groups of neurosecretory cells that transport their secretions to the corpus cardiacum.
ADVERTISEMENTS:
Sub-esophageal and ventral chain ganglia:
Neurosecretory cells are also found to be located in sub-esophageal and ventral chain ganglia. The sub-esophageal ganglia are composed of three fused ganglia that innervate sense organs, and muscles associated with mouth parts, salivary glands and neck region.
Corpora cardiaca:
The corpora cardiaca arise from the nervous system and are situated behind the brain in close association with the dorsal aorta. They receive axons from the neurosecretory cells in the brain and serves as storage and release sites for their secretions. That is why it also acts as neuro- haemal organ.
ADVERTISEMENTS:
Four cellular elements have been recognised in this organ:
(1) The bulbous endings of neurosecretory axons whose perikarya are located in the dorsum of the brain,
(2) The perikarya of neurosecretory cells that send axons into nerves that supply various peripheral organs,
(3) Glia-like cells and
ADVERTISEMENTS:
(4) Intrinsic corpus cardiacum cells.
Although, the corpora cardiaca are storage release centres, there are evidences that their own cells are capable of producing secretions.
Endocrine Organs of Epithelial Origin:
Corpora allata:
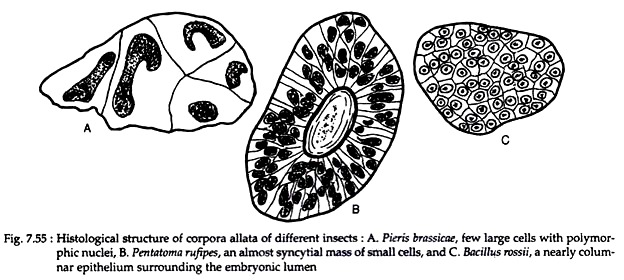
Aggregation of ectodermal cells proliferated from the surface epithelium in the vicinity of the mouth parts are seen in the posterior margin of the brain. These cells form the gland, corpora allata. These glands are commonly paired and laterally placed (Periplaneta) or they may fuse to form a single structure (Rhodnius).
In the butterfly Pieris, few large cells with polymorphic nuclei are found in corpora allata. The corpus allatus of bug (Pentatoma rufipes) consists of almost syncytial mass of small cells with cell boundaries barely distinguishable. In phasmid (Bacillus rossii), a nearly columnar epithelium surrounding the embryonic lumen persists in the adult phasmids (Fig. 7.55).
Thoracic gland:
These glands are found only in immature insects, with the exception of the Apterygotes. These are also called prothoracic glands or ecdysial glands. These glands consist of irregular masses of tissue of ectodermal origin that are usually intimately associated with tracheae.
ADVERTISEMENTS:
The glands may or may not be innervated. Depending upon its final location, these glands are also identified as peri-tracheal or ventral glands. The cells of these glands show cyclic secretory activity, reaching a maximum between moults. The glands atrophy in the adults.
Ring gland:
The larvae of higher Diptera (true flies) contain a small ring of tissue, supported by tracheae, called the ring gland or Weismann’s ring. The different cells that compose it are considered to be homologous with corpora allata, corpora cardiaca and the thoracic glands.
Some Other Endocrine Glands:
In Aeschnia cyanea, two types of endocrine cells were found to be located in midgut. One type is filled with dense granules and the other includes vesicles with an excentric core or has a loose filamentous appearance. These cells discharge their contents into the internal medium at the level of the basement membrane.
1. Neurohormones of the Brain:
Ecdysiotropin:
Protocerebrum secretes ecdysiotropin or prothoracicotropic hormone (PTTH) or brain hormone (BH) that acts on ecdysial glands.
Bursicon (Tanning hormone):
Neurosecretory cells within the brain produce a blood borne hormone which triggers the tanning or darkening of adult cuticle. It is a protein hormone with a molecular weight of about 40,000. It is released into the fused thoracoabdominal ganglia. Bursicon is released after the emerged fly has dug its way out of the soil, but in some insects this hormone may be secreted slightly before or during the loss of the old skin.
Eclosion hormone:
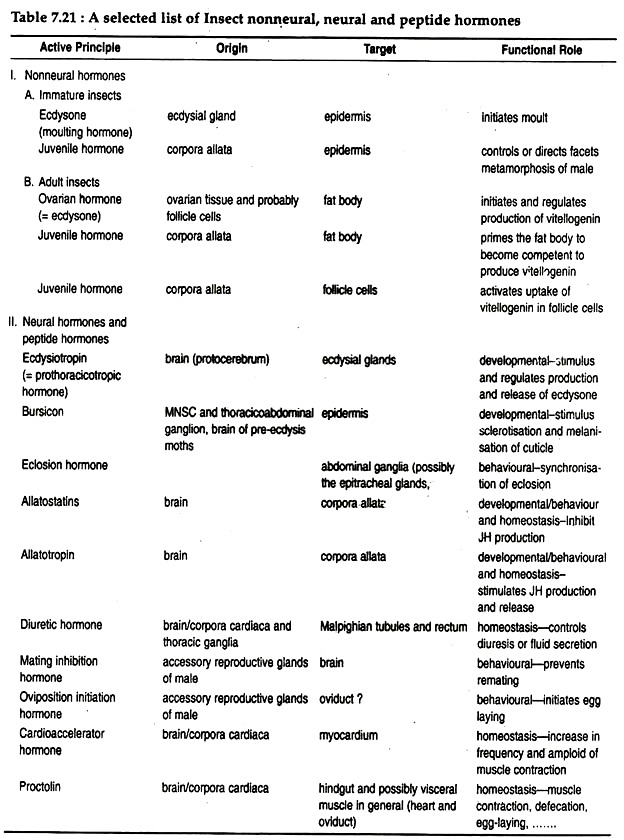
Median neurosecretory cells of the brain produce eclosion hormone, which collects in the corpora cardiaca and is released into the blood at the time of switchover from pupal to adult stage. In most insects it acts upon neurons within the abdominal ganglia to initiate the pre-eclosion behaviour. Other neurohormones released from the brain and their target organs, and their functions are mentioned in Table 7.21.
2. Hormones of Corpora Allata:
Juvenile hormone:
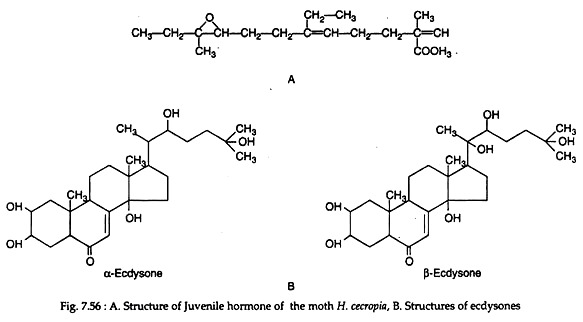
Juvenile hormone is produced by the corpora allata. Chemically the hormone is methyl 10-epoxy-7-ethyl-3, ll-dimethyl-2, 6-tridecadienoate in moth Hyalophora cecropia (Fig. 7.56A). It is likely that considerable species variability may be found in chemistry of this hormone since the corpora allata vary in functional importance.
3. Hormones of Ecdysial Gland:
Moulting hormone (Ecdysone):
Ecdysial gland secretes the moulting hormone. It is a steroid, called ecdysone. Two types of ecdysones can be synthesised by insects – α-ecdysone and β-ecdysone. The latter steroid differs from α-ecdysone only by having a hydroxyl group at position 20 (Fig. 7.56B). Similar β-ecdysone is also produced by crustacean arthropods (for functions see Table 7.21).
Functions of endocrine organs in insects:
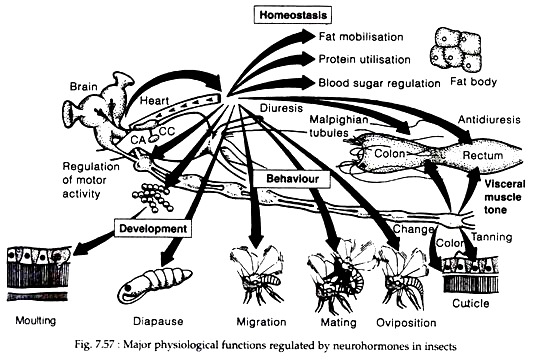
Insect hormones and neurohormones have been studied with respect to their involvement in a number of general physiological functions (Fig. 7.57). Specifically, hormones and neurohormones influence development, diapause, mating and oviposition, metabolism, development of nervous system, control of circadian rhythms, regulation of dormancy, pheromone production and regulation of migratory behaviour.
Endocrine Control of Growth and Metamorphosis:
Upon emergence from the egg, the immature insects gradually increase in size to reach adults through some mechanisms called moulting. Moulting involves the periodic digestion of old cuticle, secretion of new cuticle (usually with larger surface area than the older one) and shedding of undigested old cuticle.
The last step – shedding of undigested old cuticle- is commonly referred to as ecdysis. Sometimes the term moult is also used synonymously with ecdysis. A typical insect goes through a series of moults, increasing in size with each step. Each developmental stage of the insect itself is called an instar, and the interval of time passed in that instar is referred to as stadium.
The whole developmental process by which the first instar immature stage of an insect is transformed into the adult insect is called metamorphosis. Metamorphosis can occur slowly in some insects or abruptly in others. The insects are divided into groups based on the type of metamorphosis.
During metamorphosis apterygote insects undergo very minor changes in form, as the immature instars differ from the adult only in size and development of gonads and external genitalia. These insects are called ametabola and undergo ametabolous development. Members of pterygota can be divided into two groups exopterygota where wings develop externally and endopterygota where wings develop inside the body of larvae.
The metamorphosis of exopterygotes is referred to as hemimetabolous or simple or incomplete development where the immature instars, commonly referred to as nymph, resembles the adults in many respects, including the presence of compound eyes, but they lack functional wings, gonads and external genitalia.
The remaining group of pterygota, those classified as endopterygota, undergo the developmental process referred to as holometabolous development or complete metamorphosis.
In these insects, the immature instars are referred to as larvae, which are quite dissimilar to the adults and are adapted to different environmental situations. The larvae typically lack compound eyes, may have mandibulate mouthparts and may or may not have thoracic or abdominal legs. In these insects, the changes in the transformation from the last instar larva to the adult are compressed into a single intervening instar, called pupa.
In most endopterygotes, the larval instars resemble one another except for a few minor morphological details and in size. However, some holometabolous insects pass through one or more larval instars that are totally different from the others (e.g., some members of Coleoptera, Diptera). This is called hyper-metamorphosis.
Endocrine Control of Growth and Metamorphosis:
Hormones required:
Brain hormone, Ecdysone, Juvenile hormone.
Tissues involved:
Lateral neurosecretory cells of the brain, corpus cardiacum, corpora allata, prothoracic gland.
Hormonal involvement:
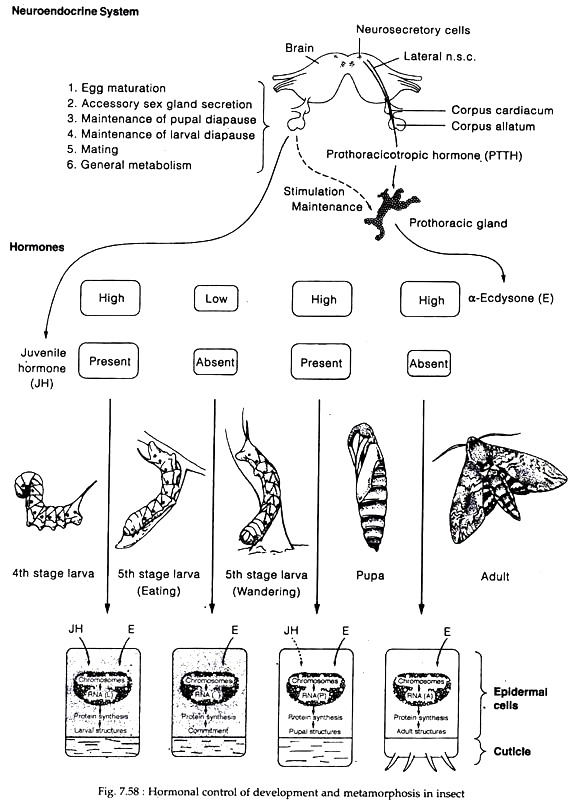
Protocerebrum secretes brain hormone (BH) or prothoracicotropic hormone (PTTH) or ecdysiotropin which accumulate in the carpora allata and subsequently released into the haemolymph (except in Lepidoptera, in other insects BH is stored in corpora cardiaca). Through the haemolymph, PTTH reach to prothoracic gland and stimulate its secretory activity.
Secretion of PTTH from brain requires different stimuli in different insects. For example, in blood-sucking bug Rhodnius, abdominal distension resulting from feeding as well as a nutritional component of the diet stimulates secretion of PTTH from the brain. In Locusta, stretch receptors on the wall of pharynx seem to be involved whereas in Lepidoptera, the increasing weight of larva was thought to be the cue.
The prothoracic glands secrete α-ecdysone or moulting hormone (MH) which through haemolymph reach the target (epidermis) and is converted into active hormone 20-hydroxyecdysone, which initiates the growth and moulting activities of the cells.
Ecdysone fovours the development of adult structures and favours the moulting processes that terminate into successive larval instars. During pupal stage, ecdysone is needed for differentiation of the adult structures and the final pupal moult.
The corpora allata secrete juvenile hormone (JH), which promote larval development and inhibit development of adult characteristics. It has been described to have a “status quo” effect, which implies that it is more of an inhibitory substance than an active growth factor (Williams).
During the early larval instars, both MH and JH are produced prior to moult. In fact, JH interacts with MH to stimulate larval maturation during each stage of development. The concentration of JH evidently decreases toward the end of a larval instar, allowing the ecdysone to cause moulting.
The total picture here should be one of balanced interaction-synergism—between these two hormones to induce normal growth and differentiation, rather than a simple antagonism.
During the last immature instar, two separate and distinct peaks of ecdysone are present in both the holometabola and hemimetabola. The first one is low and in absence of JH, the epidermal cells are reprogrammed from larval to pupal commitment in holometabolous insects (Fig. 7.58) and from nymphal to adult stage in hemimetabolous insects.
The second ecdysone peak being high causes moulting (i.e. pupa in holometabolous form) in association with JH. The presence of JH at this stage prevents the imaginal discs (which have been growing slowly in the larval stages) from developing into adult structures during the pupal stage. Finally, the next high peak of ecdysone in the absence of JH causes adult development.
In hemimetabolous insects, there is a gradual progression toward adult stage, but there are also two ecdysone peaks prior to adult moult. In holometabolous form, on the other hand, the changes from immature to adult are compressed into the pupal stage. Thus, other than the pupal stage, there are no significant differences in the basic physiological processes controlling growth and metamorphosis in either of these two groups.
Molecular mechanism:
Ecdysone acts on several genes through receptor mediated processes and as a result, influences the synthesis of a number of enzymes as well as structural proteins. It has been shown that in Chironomus larvae, ecdysone produces puffing patterns in chromosomes identical to those patterns that occur during pupation. The puffing areas are the regions of newly synthesised mRNA. Ecdysone is thought to activate a gene that directs the synthesis of key enzyme of sclerotisation process.
In Drosophila, ecdysone was shown to cause two puffing patterns. First, it combines with ecdysone receptor, which in turn, causes early puff patterns. These early puff produces proteins that activate late puff patterns and also inhibit protein synthesis of the early puffs. At the same time, the late puff proteins are initially inhibited by the ecdysone.
The mode of action of JH is not known clearly, but is suggested that the hormone may activate or repress target gene or genes by binding with a specific receptor on a regulatory sequence of the DNA. It also acts by altering the number of ecdysone receptors present in a target tissue and JH may also influence the rate of target gene transcription in both larvae and adults.
Role of other hormones in metamorphosis:
Eclosion:
Eclosion hormone or EH is released from brain by a circadian clock and declining ecdysteroid titers. If ecdysone titer is artificially kept high, the release of eclosion and its activity are inhibited. This hormone influences many aspects of pupal-adult ecdysis, including the behaviour associated with ecdysis and subsequent degeneration of abdominal inter-segmental muscles used in the act of ecdysis.
Isolation of eclosion hormone gene from different insects suggested that EH hormone as well as the mechanism by which ecdysis behaviour is triggered is conserved among insects.
Ecdysis triggering hormone:
It is the most recent hormone discovered that plays an important role in ecdysis. This 26 amino acid peptide hormone is synthesised by the epitracheal glands that are located segmentally in larvae, pupae and adults of Manduca sexta. According to Zitnan (1996), this hormone may act upstream from the eclosion hormone in a series of cascade events leading to ecdysis.
Bursicon (Tanning hormone):
Bursicon, commonly found in neurohaemal organs associated with the ventral chain ganglia is suggested to stimulate tanning and sclerotisation of the cuticle following ecdysis.
Hormonal control of reproduction:
Like other higher multicellular organisms, reproduction in insects is a camplex process. Different stages of reproduction, starting from the production of male and female gametes to oviposition, are seem to be influenced by several hormones.
(i) Spermatogenesis:
Ecdysone controls the permeability of the testis walls to the humoral factor differentiating the spermatocytes. Juvenile hormone is shown to have some inhibitory effects on spermatogenesis in many insects.
(ii) Vitellogenesis:
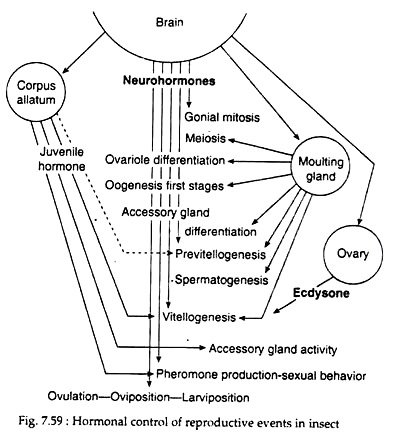
Vetellogenesis or egg yolk synthesis is also known to depend on JH from the corpora allata. In mosquitoes, juvenile hormone is required for egg development only during the early previtellogenic stages of development of the follicles. It promotes the growth of existing primary follicles up to the “resting stage”. The allatohibin (20-HE) from the ovary has two important effects.
It promotes early growth of secondary follicles leading to their separation as identifiable follicles, and is also important in initiating and sustaining synthesis and release from the fat body of vitellogenin. The third hormone necessary for ovarian follicular development is egg development neurosecretory hormone (EDNH). This peptide from the brain, controls ecdysone synthesis by ovary.
(iii) Oogenesis:
The complex process of oogenesis differs among species. However, role of brain neurosecretory cells, ecdysone, corpora allata and ovary has been postulated in general. Hormones from corpora allata help in egg maturation through the incorporation of yolk into the oocyte.
Some substances like farnesol and farnesyl methyl ether can mimic the effects of allata hormone and have a gonadotropic action. The secretions from the median neurosecretory cells of brain stimulate corpus allatum and protein synthesis (required for yolk formation).
In addition to secretions from brain cells and corpora allata, ecdysone has been found to be involved in control of oogenesis in female mosquitoes. Following a blood meal, lateral neurosecretory cells secrete egg development neurosecretory hormone, which in turn, induces the ovary to secrete ecdysone. Ecdysone, in turn triggers the synthesis of yolk protein vitellogenin in the fat bodies. Juvenile hormones secreted by corpora allata also activate fat body and ovaries.
(iv) Fertilization:
In many insects studied, ovulation (the passage of egg from the ovary into the oviduct) and oviposition, (passage of fertilized eggs to the outside, are closely linked. Both these events are affected by some peptides secreted by male accessory glands and neurosecretory products of brain. In Rhodnius, ovulation is controlled by a myotropic peptide originating in 10 identified neurosecretory cells of the pars intercerebralis.
The process of reproduction involves both the nervous and endocrine systems. The major centres are the neurosecretory cells of brain and the major events are the secretion of juvenile hormone by corpora allata, and either ecdysone production by ecdysial gland in immature insects or ecdysone biosynthesis by the ovary in adult insects. Both hormones act either independently or together in association with nervous system to make reproduction success (Fig. 7.59).
Hormonal Control of General Body Function:
Digestion and Nutrition:
In some insects movements of alimentary canal that complement the action of digestive enzymes and absorption of food is reported to be controlled partly by neurosecretory cells. In cockroaches, proctolin, a neurohormone, regulates peristalsis of hindgut. In Diptera, production and secretion of saliva are under control of a neurohormone (supposed to be 5-hydroxytryptamine, 5HT).
Circulation:
Eclosion is known to control the eclosion heart-beat. At the time of adult eclosion from the pupal case and cocoon, the eclosion hormone influences cardio acceleration, which lasts only for 75 minutes of the eclosion period.
Excretion:
The production of urine in insects is controlled by diuretic or antidiuretic hormones that are synthesised by pars intercerebralis of brain, corpus cardiacum, ventral chain ganglia and sub-esophageal ganglia. In Locusta, an antidiuretic hormone and a chloride transport-stimulating hormone have been shown to regulate both ion and water balance. In Periplaneta, proctolin is responsible for the hindgut motility which also acts as a neuromodulator helping in the excretion process.
Hormonal Control of Metabolism:
Lipid metabolism:
In most insects, lipid remains stored in the fat body, an organ often suggested to be analogous to mammalian liver. Many hormones in different insects are known to stimulate lipid content of haemolymph. The adipokinetic hormone or AKH released from corpora cardiaca and prawn red pigment concentrating hormone (PRCH), an octapeptide have considerable adipokinetic activity. Interestingly, in cockroaches, AKH has no adipokinetic effect. In Locusta, storage of lobe of corpora cardiaca produces a hypoliparmic factor that opposes the action of AKH.
Carbohydrate metabolism:
In mosquitoes, median neurosecretory cell hormone (MNCH) suppresses glycogen synthesis. Diapause hormone is suggested to stimulate glycogen synthesis during diapause. Hormones secreted by corpora cardiaca inhibit oxidation of glucose in many insects.
Protein metabolism:
In insects hormonal control of protein metabolism has not been studied in detail (except for the tanning factors). However, it is reasonable to assume that some hormones may directly or indirectly influence the metabolism of amino acids in general.
Hormonal control of behaviour:
Hormones play a very important role in insect behaviour. They either act as modifiers or as releasers of behaviour. As a modifier, a hormone alters the responsiveness of an organism. For example, the influence of ecdysone on the biting behaviour of female Anopheles, which does not take blood meals once ovarian development, has been initiated.
Ecdysone secreted by ovaries, inhibits biting when a hormone acts as a releaser, a specific behavioural pattern is exhibited within a few minutes of the secretion of that hormone. For example, ecdysis-triggering hormone and eclosion hormone in the moth Antheraea, act both as releasers in triggering the pre-eclosion behaviour and as modifiers in ‘turning on’ adult behaviour.
Migration:
Migration in some insects occurs on the basis of endocrine changes correlated with particular environmental effects, such as crowding, food deficiency, and short day. For example, in aphids, crowding results in the production of winged forms (alate) instead of wingless, non-migrant (apterous) forms.
This is associated with the activity of corpus allatum. It has also been suggested that migratory behaviours depend on a particular balance between ecdysone and juvenile hormone in the haemolymph.
Dormancy:
In response to the changes in the abiotic environment, insect development and general activity may undergo two kinds of suppression or dormancy. In response to adverse environmental condition, insects may slowdown metabolism and development; when conditions are no longer adverse, both functions resume immediately. This type of dormancy is referred to as quiescence.
Insects may enter into a state of metabolic and developmental arrest in response to certain environmental conditions which may or may not be adverse, but serve as indicators of the imminent onset of adverse condition. Development does not necessarily resume immediately with the return of favourable conditions. This type of dormancy is called diapause.
Diapause is controlled by hormones. The activity of the prothoracic gland is required for the termination of post embryonic diapause in silk moth, Platysamia. In other moths like Bombyx and Phalaenoides, a secretion from the sub-esophageal ganglia of the females induces diapause in the embryos. The arrest of development in larval and pupal diapause has classically been ascribed to an arrest of PTTH secretion.
In some species, the biological clock directly regulates PTTH secretion by a mechanism located entirely within the brain while in other species PTTH secretion may be restrained by high levels of JH. Indeed, high JH levels during larval diapause lead to the invocation of JH in regulation of PTTH release in non-diapausing moults. In some parasitic insects, development is closely attuned to the hormonal changes in the host. In these insects, diapause is induced in synchrony with diapause in the host.