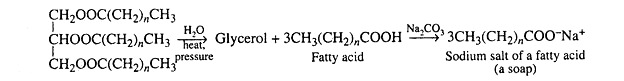In this article we will discuss about:- 1. Definition of Lipids 2. Classification of Lipids 3. Physical Properties 4. Chemical Properties 5. Special Features 6. Functions.
Contents:
- Definition of Lipids
- Classification of Lipids
- Physical Properties of Lipids
- Chemical Properties of Lipids
- Special Features of Lipids
- Functions of Lipids
1. Definition of Lipids:
Lipids are a heterogeneous group of organic compounds that are important constituents of plant and animal tissues. They are arbitrarily classed together according to their solubility in organic solvent such as benzene, ether, chloroform, carbon terachloride (the so-called fat solvents) and their insolubility in water. Their solubility properties are a function of their alkane-like structures.
Edible lipids constitute approximately 25-28% of the diet and they serve as a starting material for the production of many important commodities such as soap products. The role of lipids in the diet has received a great deal of attention because of the apparent connection between saturated fats and blood cholesterol with arterial disease. Lipids are the most important energy storage compounds in the animal kingdom.
ADVERTISEMENTS:
In contrast, plants store most of their energy in the form of carbohydrates, primarily as starch. In addition, lipids provide insulation for the vital organs, protecting them from mechanical shock and maintaining optimum body temperature. Lipids are integral components of cell membrane structure and, as such, are associated with transportation across cellular membranes.
2. Classification of Lipids:
Unlike polysaccharides and proteins, lipids are not polymers—they lack a repeating momomeric unit. However, like carbohydrates, they can be classified according to their hydrolysis products and according to similarities in their molecular structures. Three major subclasses are recognised:
ADVERTISEMENTS:
1. Simple lipids:
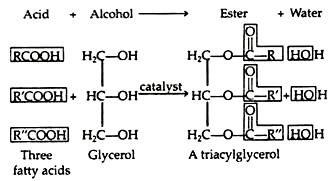
(a) Fats and oils which yield fatty acids and glycerol upon hydrolysis.
(b) Waxes, which yield fatty acids and long-chain alcohols upon hydrolysis.
2. Compound lipids:
ADVERTISEMENTS:
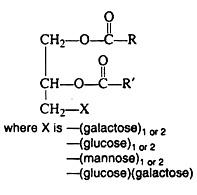
(a) Phospholipids, which yield fatty acids, glycerol, phosphoric acid and a nitrogen-containing alcohol upon hydrolysis.
(b) Glycolipids, which yield fatty acids, sphingosine or glycerol, and a carbohydrate upon hydrolysis.
(c) Sphingolipids, which yield fatty acids, sphingosine, phosphoric acid, and an alcohol component upon hydrolysis.
3. Steroids:
Compounds containing a phenanthrene structure that are quite different from lipids made up of fatty acids.
Simple lipids:
Fats and oils are the most abundant lipids found in nature. Both types of compounds are called triacylglycerols because they are esters composed of three fatty acids joined to glycerol, a trihydroxy alcohol:
Further classification of triacylglycerols is made on the basis of their physical states at room temperature. It is customary to call a lipid a fat if it is solid at 25°C, and an oil if it is a liquid at the same temperature. (These differences in melting points reflect differences in the degree of unsaturation of the constituent fatty acids.) Furthermore, lipids obtained from animal sources are usually solids whereas oils are generally of plant origin. Therefore, we commonly speak of animal fats and vegetable oils.
ADVERTISEMENTS:
3. Physical Properties of Lipids:
As previously mentioned, lipids may be either liquids or non-crystalline solids at room temperature. Contrary to popular belief, pure fats and oils are colourless, odorless, and tasteless. The characteristic colours, odours, and flavours associated with lipids are imparted to them by foreign substances that have been absorbed by the lipid and are soluble in them.
For example, the yellow colour of butter is due to the presence of the pigment carotene; the taste of butter is a result of two compounds, diacetyl (ch3cococh3), and 3-hydroxy-2-butanone (ch3cochohch3), that are produced by bacteria in the ripening of the cream. Fats and oils are lighter than water, having densities of about 0.8 gm/cm3. They are poor conductors of heat and electricity and, therefore, serve as excellent insulators for the body.
4. Chemical Properties of Lipids:
a. Saponification:
Triacylglycerols may be hydrolysed by several procedures, the most common of which utilizes alkali or enzymes called lipases. Alkaline hydrolysis is termed saponification because one of the products of the hydrolysis is a soap, generally sodium or potassium salts of fatty acids.
This hydrolysis reaction also provides a useful analytical method for the determination of a constant, the saponification number, which is characteristic of the simple lipids. The saponification number of a lipid is defined as the number of milligrams of potassium hydroxide required to saponify 1 gm. of a fat or an oil. It gives an indication of the average molar mass of the lipid. 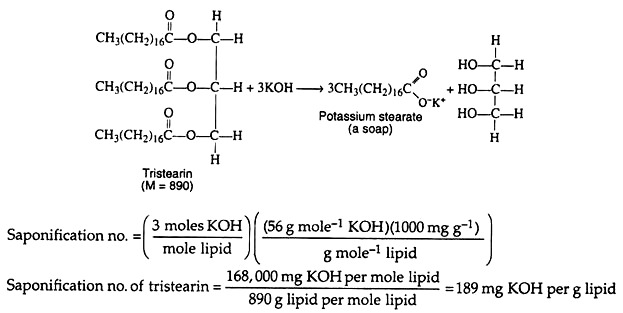
A lipid that contains long-chain fatty acids will have fewer molecules of acid per unit mass than one containing the short- chain fatty acids. Consequently, a lipid with preponderance of long-chain fatty acids will have a low saponification number in comparison to a lipid containing short-chain fatty acids.
In other words, a small saponification number for a fat or an oil indicates a high molar mass. The point is clarified by the method of determination of the saponification number of tristearin. Experimentally, a weighed sample of fat (tristearin) is saponified with a standard solution of alcoholic potassium hydroxide. Following saponification the excess alkali is determined by titration with standard acid.
b. Halogenation:
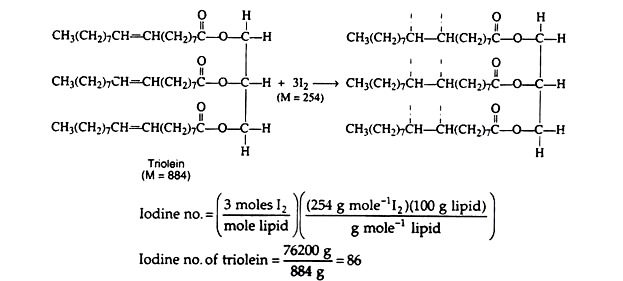
Unsaturated fatty acids, whether they are free or combined as esters in fats and oils, react with halogens by addition at the double bond(s). The reaction (halogenation) results in the decolourisation of the halogen solution.
Since the degree of absorption by a fat or oil is proportional to the number of double bonds in the fatty acid moieties, the amount of halogen absorbed by a lipid can be used as an index of the degree of unsaturation.
The index value is called the iodine number and is defined as the number of grams of iodine (or iodine equivalent) that will add to 100 grams of fat or oil. This value is influenced by a number of factors. Such as percentage of unsaturated fatty acid in the triacylglycerol molecule and the degree of unsaturation of each fatty acid.
As a general rule, a high iodine number indicates a high degree of unsaturation. Natural fats that have a preponderance of saturated fatty acids have iodine numbers of about 10-50; those that contain an abundance of polyunsaturated fatty acids have iodine numbers of 120-150.
One example is the determination of the iodine number of triolein. The equation indicates the addition of molecular iodine. In actual practice, however, the reagents used are the inter-halogens iodine mono-chloride (IC1), or iodine mono-bromide (IBr), both of which are more reactive than iodine alone.
A weighed sample of lipid is treated with an excess of the iodine reagent. After the reaction is completed, the unused iodine is determined by titration with a standard solution of sodium thiosulfate.
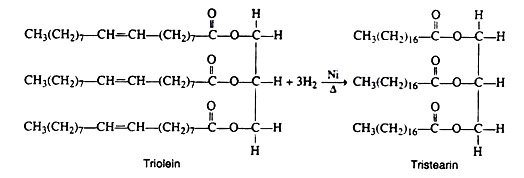
c. Hydrogenation:
A large-scale commercial industry has been developed for the purpose of transforming vegetable oils into solid fats. The process of converting oils to fats by means of hydrogenation is sometimes referred to as hardening. One method consists of bubbling hydrogen gas under pressure (25 lb/in2) into a tank of hot oil (200°C) containing a finely dispersed nickel catalyst. An example is the conversion of triolein to tristearin is given in the figure.
The equation represents the complete saturation of an unsaturated lipid. In the actual hardening process, the extent of hydrogenation is controlled so as to maintain a certain number of unsaturated linkages. If all the bonds become hydrogenated, the product becomes hard and brittle like tallow.
If reaction conditions are properly controlled, it is possible to prepare a fat with a desirable physical consistency (soft and pliable). In this manner, inexpensive and abundant vegetable oils (cottonseed, corn, and soybean) are converted into oleomargarine and cooking fats. The peanut oil in peanut butter has been partially hydrogenated to prevent the oil from separating out.
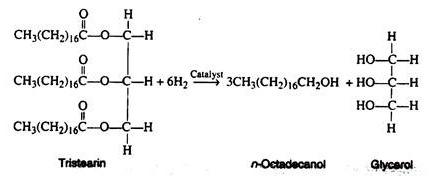
Today, because of the possible connection between saturated fats and arterial disease, many people are cooking with the vegetable oils (especially safflower seed oil) rather than with the hydrogenated products. If the hydrogenation of an oil is allowed to continue for a long period of time, glycerol and long-chain alcohols are formed e.g., tristearin to glycerol. These long chain alcohols are employed in the manufacture of synthetic detergents.
5. Special Features of Lipids:
a. Rancidity:
The term rancid is applied to any fat or oil that develops a disagreeable odour. Two principal chemical reactions are responsible for causing rancidity—hydrolysis and oxidation.
Butter is particularly susceptible to hydrolytic rancidity because it contains many of the lower molar mass acids (butyric, caproic) all of which have offensive odours. Under moist and warm conditions, hydrolysis of the ester linkages occurs, liberating the volatile acids. Microorganisms present in the air furnish the enzymes (lipases) that catalyse the process. Rancidity can easily be prevented by storing butter covered in a refrigerator.
Oxidative rancidity occurs in triacylglycerols containing unsaturated fatty acids. The reaction is quite complex, but it is believed that the first step involves the formation of a free radical, followed by production of hydro peroxides. Further reactions occur in which bonds are cleaved and the short-chain; offensive-smelling carboxylic acids are liberated.
Rancidity is a major concern of the food industry, and chemists involved in this area are continually seeking new and better substances to act as antioxidants. Such compounds are added in very small amounts (0.01-0.001%) to suppress rancidity.
They have a greater affinity for oxygen than the lipid to which they are added and thus function by preferentially depleting the supply of adsorbed oxygen. Two naturally – occurring antioxidants are vitamin E and ascorbic acid (vitamin C).
b. Drying Oils:
A drying oil is any substance that causes a paint or varnish to develop a hard, protective coating. It is the susceptibility of highly unsaturated oils to react with oxygen that accounts for their usefulness in the paint industry. Linseed oil is especially reactive and is most commonly used. The term drying may be a misnomer because it implies that the protective coating is formed by the evaporation of the solvent.
Instead, the drying process involves an oxidation followed by a polymerization reaction that results in the formation of a vast interlocking network of triacylglycerols joined by peroxide bridges. These oxidation-polymerisation reactions are catalyzed by metal ions (lead, manganese, cobalt), and salts of these metals are included in paint to hasten the drying process.
Oil paints are suspension of very finely divided pigments in linseed oil. Olicloths is made by the application of several coasts of linseed oil on woven fabric. Linoleum is a mixture of linseed oil, ground cork, and resin that has been pressed together and “dried”.
Waxes:
A wax is an ester of a long-chain alcohol (usually mono-hydroxy) and a fatty acid. The acids and alcohols normally found in waxes have chains of the order of 12-34 carbon atoms in length. Waxes are easily melted solids that are widely distributed in nature and are found in both plant and animal matters. They are not as easily hydrolysed as the triacylglycerols and therefore are useful as protective coatings.
Plant waxes are found on the surfaces of leaves and stems and serve to protect the plant from dehydration and from invasion by harmful organisms. Carnauba wax, largely myricylcerotate, C25H51COOC30H61, is obtained from the leaves of certain Brazilian palm trees and is used as a floor and automobile wax and as a coating on carbon paper.
Animal waxes also serve as protective coatings. They are found on the surface of feathers, skin and hair, and help to keep these surfaces soft and pliable. Beeswax, which is mostly myricyl palmitate, C15H31COOC30H61, is secreted by the wax glands of the bee. Spermaceti wax, mainly cetyl palmitate, C15H31COOC16H33, is found in the head cavities and the blubber of the sperm whale.
Spermaceti crystallizes in heavy white flakes when whale oil is exposed to air and chilled. It is used primarily in ointments, in cosmetics, and in the manufacture of candies. Lanolin, obtained from wool, is a mixture of fatty acid esters of the steriods lanosteriol and agnosterol. It finds widespread medical applications as a base for creams, ointments, and salves.
Compound Lipids:
a. Phospholipids:
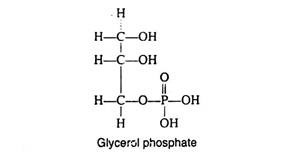
The phospholipids, also called phosphatides, are compound lipids that are derivatives of glycerol phosphate:
Phospholipids are found in all living organisms. Regardless of their source, they have quite consistent structures. Phospholipids are particularly abundant in liver, brain and spinal tissue and are found in the outer membranes of most cells. They appear to be essential components of cell structure since the amount of phospholipids present in animal tissues remains relatively constant, even during starvation when the cell’s supply of simple lipids is depleted.
Phospholipids are large molecules containing both a polar and a nonpolar component. They are the most polar of all the lipids. It is believed that their primary function is to act as an emulsifying agent at cell membrane surfaces, where water-insoluble lipids and water-soluble materials (such as proteins) must be capable of intimate association.
It is thought that phospholipids take part in fat metabolism by promoting the transportation of lipids in the blood stream, primarily in an aqueous medium. Phospholipids also play important roles in the electron transport system in secretory processes, and in the transport of ions across cell membranes. There is increased speculation regarding their functions in brain and nervous tissue, but till now their exact purpose is not known.
Two commonly found phospholipids (lecithin and cephalins) are described here:
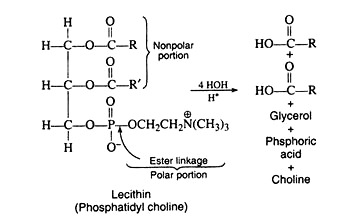
1. Lecithin:
Lecithin is probably the most common of the phospholipids. It contains the important quaternary ammonium salt choline. HOCH2CH2N+(CH3)3, joined to a phosphoric acid residue by means of an ester linkage. The nitrogen in choline carries a positive charge and the phosphate a negative charge so that in solution at most pH values, lecithin exists as an internal salt or zwitterion.
The structure and hydrolysis products of lecithin are:
Pure lecithin is a waxy white solid that quickly darkens when exposed to air. In contrast to fats and oils, it is colloidally dispersed in water and is insoluble in acetone. It is, therefore, possible to separate licithin from an ether extract by the addition of acetone. Lecithin is especially abundant in egg yolk and soybeans. When obtained from the latter source it is used as an emulsifying agent in the dairy and confectionery industries.
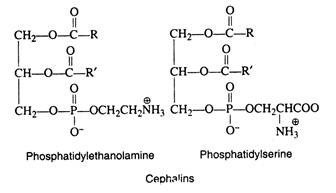
2. Cephalins:
The chief difference between the cephalins and lecithins lies in the nitrogenous base component that is linked to the phosphate moiety. In the cephalins, the choline is replaced by ethanolamine, HOCH2CH2NH2, or by the amino acid serine, HOCH2CH(NH2)COOH. The term cephalin is derived from its chief occurrence in the body, namely the head and spinal tissue (Greek, kephalikos, head). It is thought that cephalins play an important role in the process of blood clotting:
b. Glycolipids:
Several groups of compounds are found containing both lipid and carbohydrate moieties. Those that are water soluble are termed liposaccharides and are thought of as derived carbohydrates. Those that retain solubility in nonpolar organic solvents are classed as glycolipids. One group of glycolipids contains fatty acids, glycerol, and various carbohydrates.
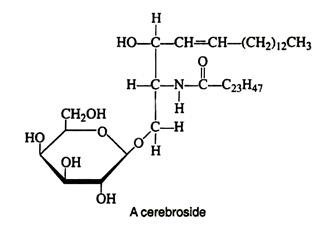
Another group, the cerebrosides, are sphingosine derivatives and, thus, may be classified either as glycolipids or sphingolipids. Cerebrosides occur primarily in the brain (7% of the solid matter) and in the myelin sheath of nerves. It has been suggested that they function in the transmission of nerve impulses across synapses.
They are also believed to be present at receptor sites for acetylcholine and other neurotransmitters. The fatty acids found in cerebrosides are unusual in that they contain 24 carbon atoms. Cerebrosides most often contain D-galactose attached by an acetal linkage at carbon-1 of sphingosine. Unlike most lipids, they are insoluble in ether but may be extracted into warm alcohol or pyridine.
Two severe lipid storage diseases are caused by errors in the metabolism of the glycolipids. In Gaucher’s disease, the glycolipids contain glocose instead of galactose. These abnormal glycolipids accumulate in the brain, spleen and kidney cells. An infant with Tay-Sachs disease lacks an enzyme that breaks down glycolipids, so they accumulate in the tissue of the brain and the eyes.
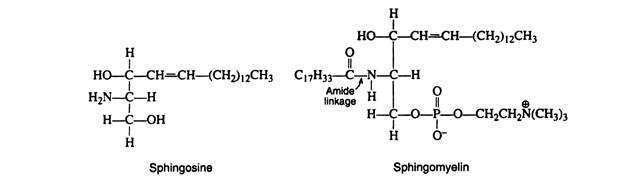
c. Sphingolipids:
Sphingolipids occur in the membranes of both plants and animals, with only a minor amount found in depot fat. They- contain the long-chain unsaturated amino alcohol sphingsine instead of glycerol. Also present are fatty acids, phosphate, and an alcohol component. The most abundant sphingolipid is sphingomyelin, which contains choline as the alcohol group.
Niemann-Pick disease is another lipid storage disease in which sphingo myelins build up in the brain, liver, and spleen, resulting in mental retardation and early death.
6. Functions of Lipids:
It is established that lipids play extremely important roles in the normal functions of a cell. Not only do lipids serve as highly reduced storage forms of energy, but they also play an intimate role in the structure of cell membrane and organellar membranes. Lipids are not transported in the free form in circulating blood plasma, but move as chylomicrons.
Chylomicrons are very low density lipoproteins, which can be absorbed from the intestinal lumen easily. Lipids also move through the circulation as free fatty acid-albumin complexes.
Lipids also participate in metabolic activities directly or indirectly. These can be described as follows:
1. Lipids are major sources of energy in animals and high lipid-containing seeds.
2. Activators of enzymes:
There are many enzymes, which require lipid micelles for maximum activation, e.g., three microsomal enzymes, namely, glucose-6-phosphatase, stearyl CoA desaturase and ω-mono oxygenase, and β-hydroxy butyric dehydrogenase (a mitochondrial enzyme), require phosphatidyl choline micelles for activation.
3. Components of electron transport chain (ETC):
The ETC in the inner membranes of mitochondria is buried in a phospholipid substrate.
4. Acts as substrate:
α-Acyl-β-oleyl phosphatidyl choline specifically serves as the acceptor of a CH3 group from S- adenosyl methionine, which adds across the double bond of β-oleyl moiety to form the cyclopropane function of lactobacillic acid.
5. Lipids as glycosyl carrier:
The isoprenoid compound, undecaprenyl phosphate, acts as a lipophilic carrier of a glycosyl moiety in the synthesis of bacterial cell wall lipopolysaccharides and peptidoglycans.