In this article we will discuss about:- 1. History of Polymerase Chain Reaction 2. Basic Polymerase Chain Reaction 3. Steps 4. Other Schemes 5. Advantages 6. Disadvantages 7. Application.
History of Polymerase Chain Reaction (PCR):
In the mid-1980s, an important revolutionary technique of molecular biology— PCR or Polymerase chain reaction—was first described. Since then, it has been modified and applied variously. PCR is a rapid versatile in vitro method for amplifying defined target DNA sequences present within a source of DNA.
Usually this method is designed to permit selective amplification of a specific target DNA sequence(s) within a heterogeneous collection of DNA sequences, e.g., total genomic DNA or a complex cDNA population. To permit such selective amplification, no transformation of bacterial cell is necessary, but one needs to have base sequence information, at least of the two terminal regions of the DNA fragment to be amplified.
Kary Mullis has been awarded the Nobel Prize in 1993 for developing such procedure by successfully exploiting a very simple and straightforward idea that has proved to be extremely useful with far-reaching consequences. This powerful technique of cell-free method of DNA cloning may replace completely the gene cloning with vectors in due course of time.
Basic Polymerase Chain Reaction:
ADVERTISEMENTS:
In living cells, the DNA replication involves polymerisation of nucleotides with the help of DNA polymerase enzyme that uses a DNA strand as template. This reaction requires a primer strand which is a small single stranded RNA molecule (synthesised with the help of RNA polymerase) to which nucleotides are added.
In PCR, similar reaction takes place without living cells in the tube with the help of special heat stable DNA polymerase enzyme. Here, the primer strand in the form of oligonucleotides, special heat-stable DNA polymerase enzyme and DNA precursors are added from outside.
Requirements:
1. DNA polymerase:
ADVERTISEMENTS:
The DNA polymerase used in PCR should be heat stable. The widely used DNA polymerase is Taq DNA polymerase, obtained from the bacterium Thermus aquaticus, inhabiting in hot spring. It is thermo-stable up to 94°C with an optimum working temperature of 80°C.
Other polymerases include Pflu DNA polymerase, obtained from Pyrococcus furiosus, Vent polymerase, isolated from Thermococcus litoralis, PWO polymerase from Pyrococcus woesi, Tma polymerase from Thermotoga maritima etc.
2. Primers:
For PCR, two oligonucleotide primers, which can base pair with the two strands of DNA on the two sides of the specific region to be amplified, are required. These primers are often about 15-25 nucleotides long. In all PCR, the specificity of amplification depends on the extent to which the primers can recognise and bind to sequences other than the intended target DNA sequences.
ADVERTISEMENTS:
3. Deoxynucleoside triphosphates:
Four types of DNA precursors viz, dATP, dCTP, dGTP and dTTP which are complementary to the individual DNA strands of target DNA segment.
4. Thermal cycler:
It is an automated cycler, first devised by Cetus Company, USA, with a built-in microprocessor-controlled temperature cycling needed for PCR.
Steps Involved in PCR:
1. Isolation and Purification of the DNA segment to be amplified:
Before starting PCR, the DNA segment or gene to be amplified is isolated from the genome or cloned. Now a day’s complete genome sequence is available for many organisms. The purity of this DNA segment or gene must be checked before starting PCR following techniques like electrophoresis.
2. Cyclical amplification of DNA:
The amplification process involves three steps. Before starting amplification reaction, the final solution taken in microfuge tube should contain desired volumes of polymerase enzyme, target DNA segment, four types of dNTPs, RNA segment (primer) and buffer. The tubes containing the PCR materials are then subjected to following reaction steps in the thermal cycler.
ADVERTISEMENTS:
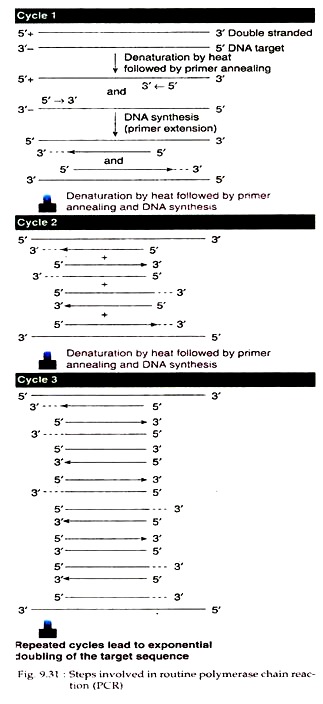
(a) Denaturation:
The thermal cycler is first adjusted to temperature at 94°C (93°-95°C for human genomic DNA). The double stranded DNA is heat denatured at this temperature and made single stranded (Fig. 9.31).
(b) Annealing:
Annealing temperature varies usually from 50°-70°C depending on the Tm of the expanded duplex and accordingly adjusted in the thermal cycler. The denatured solution is then allowed to cool to this temperature. The primers in the mixture recognise the two strands and border the sequences to be amplified. The primers then bind to their complementary strands through annealing.
(c) Chain elongation:
The annealed mixture is then incubated at 72°C for 3 minutes (adjusted in the thermal cycler). At this temperature, the polymerase enzyme synthesise new DNA strands by adding dNTPs from the 3′ OH ends of the primers. Thus, each cycle of polymerase chain reaction ends in one-round replication of the target segment of DNA.
By PCR, the amount of new DNA generated increases geometrically. Starting with one molecule of DNA, one cycle of PCR produces two molecules, two cycles produce four molecules, in this way, and ten cycles produce 1024 copies (210) of the target DNA. In the thermal cycler this process is automatically repeated 20-30 times, so that within a short time, billion copies of the DNA segments flanked by left and right primers can be produced.
In order to continue the amplification process, the temperature of the PCR mixture is alternately increased for denaturation and decreased for annealing once every 1-3 minutes (adjusted by the computerized device built in the thermal cycler). As a result, during temperature rise, the catalytic activity of DNA polymerase is not destroyed and there is no need to add a fresh aliquot of enzyme in each cycle of amplification.
3. Screening of amplified DNA:
The amplified DNA can then be separated from other DNAs (if present in the mixture) by agarose gel electrophoresis. The specificity of the amplified DNA can then be tested by Southern blot technique.
Other Schemes of PCR:
In addition to the basic PCR technique, as mentioned above, there are several variations in this technique:
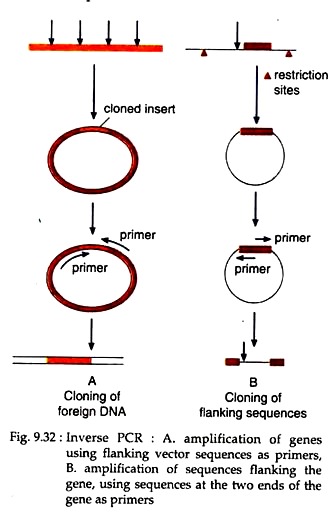
1. Inverse PCR:
If the border sequences of a DNA are not known, then that DNA segment can be amplified by inverse PCR. Here, the target DNA segment is first cloned in a vector of known sequences. The border sequences of the vector then can be used as primers in such a way that the polymerisation proceeds in inverse direction i.e., away from the vector sequence flanked by the primers and towards the DNA sequence of inserted segment (Fig. 9.32).
In this way, when the sequence of a gene is known, its border sequence can be used as primers to amplify the sequences flanking the gene (Fig. 9.32).
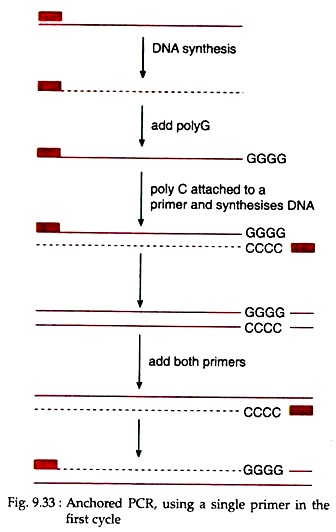
2. Anchored PCR:
For basic PCR, two primers of known sequences are used. But when knowledge about sequences at only one of the two ends of the target DNA is available then the PCR, carried out utilizing only one primer is called anchored PCR.
Here, due to the use of only one primer, only one strand of the DNA duplex will be copied first. Following first cycle, a poly G tail is added at the other end of the newly synthesised strand (Fig. 9.33). Then anchor primer attached with poly C sequence is added to the PCR mixture.
The DNA strand with poly G tail at its 3′ end then act as the template for the synthesis of DNA strand utilizing the primer linked with poly C sequence, which is complementary to the poly G of the template. Therefore, in the subsequent cycles, both the original primer and anchored primer will be utilised for the amplification of target DNA.
3. DOP-PCR (Degenerate Oligonucleotide Primed PCR):
It is a type of PCR which is designed to permit possible amplification of several products. The two primers may be partially degenerated oligonucleotides, composed of panels of oligonucleotide sequences that have the same bases at certain nucleotide positions, but are different at others.
As a result, there may be comparatively many primer binding sites in the source of DNA. This provides a means of searching for a new or un-characterised DNA sequence that belongs to a family of related sequences, either within or between species. DOP-PCR also permits indiscriminate amplification of target- DNA. Primers with random sequences can bind to numerous locations in the template DNA and permits a kind of whole genomic amplification.
4. Differential PCR or Competitive PCR:
Sometimes quantification of the number of copies of a gene is required. Due to extreme sensitivity of PCR reaction, this can be achieved by adding known amount of reference DNA into the sample, carrying out the PCR reaction on the combined DNA and asserting the amount of the product formed from it. This process is known as competitive or differential PCR.
The reference DNA is so designed so that its size should be same as that of the target DNA and a unique restriction site is incorporated in this DNA to distinguish its product from the product of the target DNA. Following PCR, both the products will be treated with the restriction enzyme. The product of the target would remain unaffected, while the product of reference DNA will be cleaved. The products can therefore be separated after PCR reaction and assayed separately.
5. RT-PCR (Reverse Transcriptase PCR):
This highly sensitive PCR technique involves two steps:
(i) In the first a cDNA is synthesised from RNA using a primer.
(ii) In the second step this specific cDNA is amplified by PCR. RT-PCR is used either for detecting the presence of a particular RNA or for quantifying the amount of an RNA.
A new version of RT-PCR is real time RT- PCR. In this process, the reverse transcriptase step is carried out as usual to produce the first strand cDNA copy of the mRNA. The DNA amplification step is then carried out in presence of SYBR green, a very sensitive fluorescent dye that stains double- stranded DNA.
As the DNA is amplified by PCR, the staining can be measured in real time (thus the name is real time RT-PCR) by means of a special thermal cycler that uses laser detection of the fluorescence. The rate of production of stained amplified DNA, compared with controls of known amounts of starting mRNA allows the detection of amount of mRNA in the experimental sample. Thus, it allows much more accurate quantification of mRNA levels.
Advantages of PCR:
The major advantages of PCR as an in vitro cloning method over the cell-based cloning method are its:
(1) Rapidity,
(2) Sensitivity, and
(3) Robustness.
1. Rapidity:
A typical PCR technique involves 30 cycles, each with three steps, viz., denaturation, annealing and chain elongation. In an automated thermal cycler, an individual cycle takes 3-5 minutes. This compares favorably with the time required for cell-based DNA cloning which may take weeks (Table 9.10).
However, some time is required for designing and synthesizing the oligonucleotide primers but this has been simplified by the availability of computer software for primer design and rapid commercial synthesis of custom oligonucleotides.
Comparison of PCR and cell-based DNA cloning
PCR (cell free DNA cloning)
1. In vitro method of target DNA amplification.
2. PCR requires very little amount of DNA [nanogram (ng)] to start.
3. PCR requires target DNA, heat-stable DNA polymerase enzyme, primers,buffer and thermal cycler.
4. Time required for a typical experiment is about 4 hours.
5. Target DNA can be taken from any source.
6. PCR requires less labour and user’s less skill.
Cell-based DNA cloning
1. In vitro method of target DNA amplification.
2. Requires microgram (µg) of DNA to start.
3. Requires restriction enzymes, ligase enzyme, cloning vectors, growth media etc.
4. Time required for a typical cell-based cloning is 3-5 days.
5. Target DNA can be taken from fresh (living) sources.
6. Cell-based cloning requires labour and high skill.
2. Sensitivity:
A very minute amount of DNA even the DNA from a single cell can be amplified by PCR. However, this extreme sensitivity of this method needs a great care to be taken to avoid contamination of the sample DNA under investigation, by other external DNA. Due to extreme sensitivity PCR has found its application in forensic science, in medical or scientific diagnosis, in genetic linkage application etc.
3. Robustness:
It means PCR allows amplification of DNA taken from samples that were not preserved properly and from which conventional DNA isolation is not possible (Table 9.10). DNA from formalin- fixed tissue samples or samples that are badly degraded can be used in PCR. As a result, PCR is very suitable for molecular anthropology and paleontology studies.
Disadvantages of PCR:
Despite its huge popularity or wide application, PCR has some limitations:
1. Prior knowledge on target DNA sequence:
In vitro amplification of DNA sequences by PCR is based on proper primers which require prior sequence information. It means that the DNA region of interest has been partly characterised previously often following cell based cloning of DNA.
However, previously un-characterised DNA sequence can also be cloned by PCR with degenerated oligonucleotides if they are members of a gene or repetitive DNA family, at least one of whose members has previously been characterised.
Sometimes, PCR is used without prior sequence information to amplify target DNA sequences indiscriminately from a source of DNA, where the target DNA is present in extremely limited quantities. So, PCR cannot have the advantages of cell-based cloning in offering a separating way the individual DNA clones, comprising a genomic DNA library.
2. Limited size and quantity:
Unlike cell-based cloning of DNA where the size of cloned DNA can be as long as 2 Mb, the reported DNA sequences that can be cloned by PCR may be 0.1-5 kb in size. Recently, amplification of longer targets of 42 kb product from the bacteriophage λ genome has been reported. However, small segments of DNA can easily be amplified by PCR. The amount of PCR product obtained in a single reaction is also much less than the amount that can be obtained using cell-based cloning.
3. Infidelity of DNA replication:
The fidelity of DNA replication in vitro is extremely high because of proof-reading mechanism of DNA polymerases. But, when DNA is replicated in vitro by PCR, the copying error rate is considerably greater due to lack of proof-reading activities of heat stable polymerases used in PCR. Recently, this problem of infidelity by PCR has been considerably reduced by the use of alternative heat-stable DNA polymerases having 3′-5′ exonuclease activity.
For example, Pyrococcus furiosus DNA polymerase is now widely used because of its proofreading conferred by its associated 3′-5′ exonuclease activity. The PCR products using this enzyme have been shown to have much lower level of mutations introduced by copying error.
From the above discussion it appears that the amount of material that can be cloned by PCR is limited. Further, this process is time-consuming and expensive. Therefore, to get large quantities of desired DNA fragment, it is suggested for cloning of a PCR product in a cell-based cloning system.
Various plasmid cloning systems are now available to propagate PCR-cloned DNA in bacterial cells. Following such cloning, the insert can be cut out with suitable restriction enzyme and transformed into other expression vectors.
Application of PCR:
In spite of some limitation, the simplicity and versatility of PCR, made it the most ubiquitous of molecular genetic methodology with a wide range of general application.
Some are:
1. Cloning of a gene:
Today complete sequence of many genomes is available for many organisms. Any gene of such an organism can be cloned without having a gene library. From the sequence data it is possible to collect information on the base sequence of the two flanking primers required for PCR. Then from a tiny amount of tissue of that organism, DNA could be isolated and subjected to PCR. The genes in question would be amplified from the total DNA.
2. Rapid genotyping for polymorphic markers:
PCR can type restriction site polymorphisms (RSPs) by simply designing primers using sequences which flank the polymorphic restriction site, amplifying from genomic DNA, then cutting the PCR product with appropriate restriction enzymes and separating the fragments by agarose gel electrophoresis.
PCR can be used to study RFLP and RAPDs. Short tandem repeat polymorphisms (STRPs) or microsatellite markers can also be typed conveniently by PCR. In this case, primers are designed from sequences known to flank a specific STRP locus, pre-mitting PCR amplification of alleles whose sizes differ by integral repeat units.
The PCR products can then be fractionated by polyacrylamide gel electrophoresis. The PCR normally includes a radioactive or fluorescent nucleotide precursor which becomes incorporated into the small PCR products and facilitate their detection.
3. Assay of Mutations:
(i) Small insertions or deletions [e.g., three nucleotide deletion in cystic fibrosis (CFTR) allele], can be detected by PCR through designing the primers from regions closely flanking the mutation site and distinguishing the normal and mutant alleles by size on gels. If the mutation includes a restriction site, mutant and normal alleles can be distinguished by amplifying the across mutant site and digesting the PCR product with an appropriate restriction enzyme.
(ii) If mutation does not result in a restriction site differences, the normal and the mutant allele can be distinguished by PCR. In this case, a primer is deliberately designed from sequence immediately adjacent to, but not encompassing the restriction site. The primer is also designed to have a mismatched nucleotide which together with the sequence of the mutant site creates a restriction site not present in normal alleles.
(iii) Oligonucleotide primers can be designed so as to discriminate between target DNA sequences that differ by a single nucleotide in the region of interest. This is a type of allele-specific PCR. In this case, allele specific oligonucleotide primers are designed to differ at the nucleotide that occurs at the extreme 3′ terminus.
It is so done because the DNA synthesis step in a PCR reaction is absolutely dependent on correct base-pairing at the 3’end. This method can be used to type specific alleles at a polymorphic locus, but was shown to be useful for detecting a specific pathogenic mutation, the so-called amplification refractory mutation system (ARMS).
4. PCR in gene therapy:
In gene therapy, the transfer of gene is done in presence of a marker gene e.g., neomycin resistance (neor), which could be detected in the patient’s blood even after 60 days. In this case, PCR is used to detect the presence of that gene. Thus, PCR helps in monitoring a gene in gene therapy experiments.
5. Prenatal diagnosis by PCR:
Prenatal diagnosis of some genetic diseases like sickle cell anemia, phenylketonuria, β- thalassemia, haemophilia etc. can be done by PCR in a very short time. In such case, PCR product of target gene is examined using a labeled probe, suggesting whether or not the mutant sequence, causing the disease is present or not.
6. Sex determination:
PCR can be utilised to study the DNA sequences in a single cell. Therefore, sex of human livestock embryos fertilized in vitro can be determined before implantation by PCR. In this case, primers and probes, specific for sex chromosome (e.g., ZFY or ZFX sequences), should be used. Today, X-specific primers and probes in humans are available. PCR can also be used for the detection of sex-linked disorders in the fertilized embryos.
7. PCR in DNA finger printing:
DNA finger printing is widely applied in forensic science. It may be achieved through PCR utilizing primers that are designed on the information of well characterised sequences of microsatellites. Such PGR can amplify DNA taken from individual hair, stains of blood or sweat having partially degraded DNA, which could not be used earlier for characterisation as well as identification of individuals.