In this article we will discuss about the preparation of permanent mounts.
1. Fresh Water Protozoans:
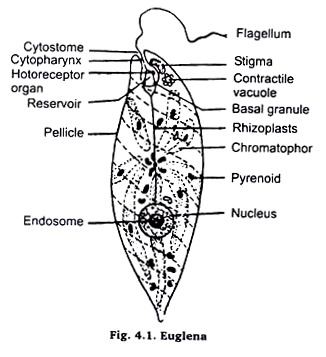
Euglena:
Living Euglena, for study, may either be collected from a patch of stagent water having decaying green leaves or may be obtained from cultures in laboratory. For purpose of study in the living condition take a few euglena in a dropper or a micro-pipette either from the culture, which is maintained in laboratory or from the bottom of a small patch of stagnent water where leaves of plants etc. are decaying.
Now pick a clean slide and put a drop of Mayer’s albumen on top of it. Rub this albumen with index finger on whole of the slide top (surface) in order to make a smooth, thin and homogeneous film.
ADVERTISEMENTS:
Now, put a drop of water, containing material, collected from pond, in the centre of slide on the same side the film is spread. Now, observe the movements & irregular shape of the animal under microscope in low magnification. Draw various stages of locomotion at an interval of half a minute.
For detailed anatomical study a permanent stained mount may be prepared by following procedure:
Take the same slide after completing the study in living condition. Now take a small piece of filter paper or blotting paper and put its one edge in contact with the fluid having Euglena on the slide. As a result of this the excess of fluid shall be absorbed by paper and the Euglena present in it shall stick to the surface of slide through Mayer’s albumen film.
ADVERTISEMENTS:
Now, kill and fix these Euglena either by putting the slide in inverted position over the mouth of asmic acid bottle in such a way that the Euglena could be exposed to fumes of osmic acid, or put a drop of 100% alcohol or 1% acetic acid or Shaudin’s fluid over the sticking Euglena.
Now, wash the slide in tap water if osmic acid or acetic acid is used, otherwise bring the slide through descending grades of alcohol (90%, 70%, 50%, 30%) to finally tap water, if 100% alcohol or Shaudin’s fluid is used. After washing thoroughly with water stain with Borax carmine, dehydrate in 50, 70, 90 and Absolute alcohyl, clear in xylol and mount in Canada Balsam. Observe under microscope.
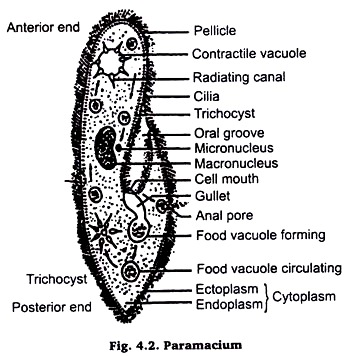
Paramaecium:
Methods are just the same as described for Euglena. The only precaution to be taken is that before putting on slide, the drop of culture fluid having animals, put a drop of weak gelatin solution or 1% agar solution or a few fibres of cotton wool in order to check the fast movement of these animals. Picro-indigo carmine or Picro-carmine or Borax-carmine stain should be used for colouring.
2. Rectal Ciliates of Frog:
The term rectal ciliates is used for all those protozoans which belong to class ciliophora and which live permanently as entoparasite in the rectum and large intestine of frog (amphibians). These are usually three in number i.e., Opalina, Balantidium and Nyctotherus. It is not necessary that all three could be available from every frog.
It is just possible that none of them might be available or only one of them may be available in a particular frog. For purpose of extraction a living frog is anesthetized by chloroform or through pithing or asphyxiation. The rectum and lower part of intestine are taken out from the frog and are cut open in a watch glass.
Their contents are thoroughly washed with phisiological saline (0.7% NaCl) and are collected in the watch glass. Now take a clean slide and apply thin film of Mayer’s albumen on top surface of it. Put a drop of the contents, from the watch glass in middle of the top surface of slide and observe under low magnification.
Find out whether ciliates are present or not. If ciliates are present absorb the excess water with a piece of filter paper or blotting paper and fix the animals by using 100% alcohol or Osmic acid bottle in the same way as in case of Euglena.
Now, bring the slide to water and use double staining method (Helematoxylene and Eosin). Dehydrate, clear in Xylene and mount in Canada Balsam.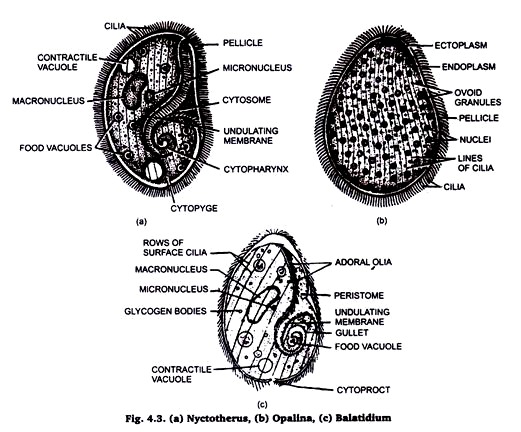
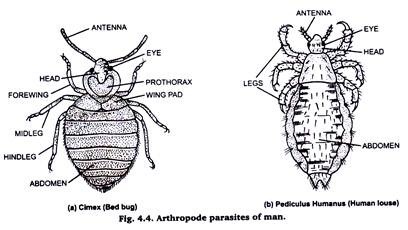
3. Arthropode Parasites of Man:
(i) Cimax:
Take a cimex (Bed bug) and kill it by chloroform and fix it in formalin. Remove formalin by washing it with water. Then keep it in a small test tube containing 10% KOH solution over-night. Then wash it thoroughly with water to remove KOH. Thereafter, dehydrate it through the grades of alcohol (staining stage may be omitted). Clear in xylol and mount in canada balsom or D.P.X.
(ii) Pediculus:
Take a pediculus (head louse) Pediculus humanus capitis or a body louse pediculus humanus corporis) and kill it by chloroform. Fix it in formalin, wash in water to remove the fixative. Put it in 5% KOH solution for 10-15 minutes and then take it out from KOH.
ADVERTISEMENTS:
Wash it again in water so that KOH is completely removed. Thereafter, dehydrate it by passing through the grades of alcohol (staining stage may be omitted). Clean in xylol and mount in Canada Balsom or D.P.X, Then study under microscope.
4. Fresh Water Annelids:
Several slides have been prepared from fresh water Annelids e.g. Earthworm, Nereis and Leach.
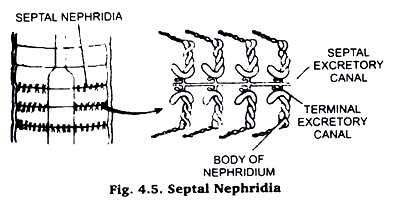
(i) Septal nephridium of Earthworm:
Take a preserved earthworm. Stretch and pin it in a dissecting tray. Now, cut it along mid-dorsal line behind the clitellum. Take a small piece of septum, which extends from alimentary canal up to body wall.
Wash it thoroughly with water, dehydrate up to 70% alcohol, stain in Borax carmine and again dehydrate through 90% and absolute alcohol, clear in xylol and mount in Canada balsam. For isolating only one nephridium use two sharp needles and operate on the slide under binocular. (Fig. 4.5).
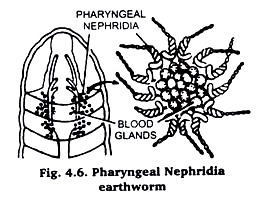
(ii) Pharyngeal nephridia from Earthworm:
Pharyngeal nephridia are present in the region of pharynx in 4th to 6th segments. They appear as a bunch of tubules in the inter segmental space above the pharynx. Cut open the earthworm in the aforesaid region and pick a small part of the bunch.
Ascertain under microscope. When satisfied, wash with water, dehydrate thoroughly in 30%, 50%, 70% alcohol, stain in Borax carmine dehydrate through 90% and absolute alcohols, clear in Xylol and mount in Canada balsam Fig. 4.6.
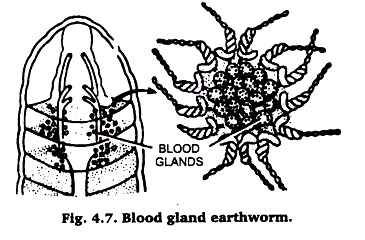
(iii) Blood glands from Earthworm:
They are found mixed with pharyngeal nephridia. They are dark brown, small rounded structures. Proceed as for pharyngeal nephridia above. (Fig. 4.7).
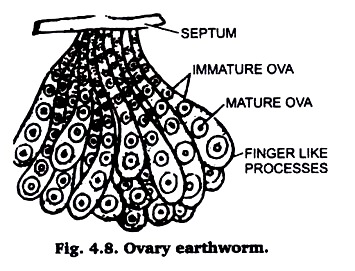
(iv) Ovary of Earthworm:
Take either a preserved or a fresh and earthworm. Stretch and pin it in a dissecting trey. Now, make a cut in the mid dorsal line anterior end. The two flaps, so cut open, should be transversely after 15th segment. Now, pull the alimentary canal upwards and towards holding its cut hind end. Pulling of the alimentary canal should be slow and gradual.
As soon as you reach the anterior edge of clitellum, near septum between 12th and 13th segments, hold the alimentary canal vertically. Near the septum along the mid ventral line of alimentary canal look for a pair of dark blackish brown hearts. Now, search for two tiny white dots, which may be found either attached to hearts or attached to integument just close to hearts.
With the help of the forceps take out both these ovaries and put them on slides on which a thin film of Mayer’s albumen has been applied. The albumen will hold the ovaries in place and will not let them watched away with reagent. When slightly dry, wash in tap water stain with borax carmine after dehydrating up to 70% alcohols and pass through 90% and absolute alcohol, clear in xylol and mount in Canada balsam Fig. 4.8.
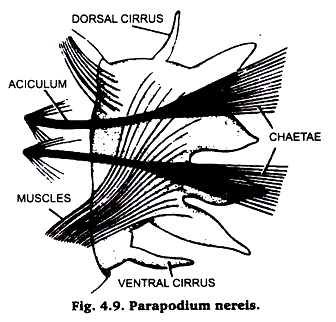
(v) Parapodium from Nereis:
For the preparation of slides of parapodia instead of complete animal, only one or two segments shall be provided to each student. The parapodia are lateral extensions of segmental skin.
With the help of scissors cut the parabodium near its base, wash thoroughly with water, dehydrate up to 70% alcohol, stain with Borax carmine, completely dehydrate in 90% and absolute alcohols, clear in Xylol and mount in Canada balsam Fig. 4.9.
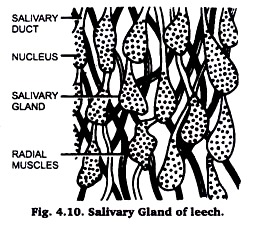
(vi) Salivary glands of Leech:
Take a fresh narcotized leech. Put it in a patri dish and press the animal from behind forward. This process will remove the blood from the alimentary canal. Repeat this process two to three times or till the blood stops coming out of its mouth.
Now pin up the animal in a dissecting tray after stretching the animal with ease as far as possible. Now, with the help of scissors or with the help of a blade make a cut in the anterior one inch of the animal along the mid dorsal line up to the position of pharynx. With the help of a scalpel remove the muscular and botryoidal tissue from the body wall and pin down the two flaps of the bodywall in the dissecting tray.
Locate the buccal cavity and try to search the salivary glands among the radial muscles which extend from the buccal cavity and pharynx to the body wall. Pick up a bunch of few of these glands and observe under low power of microscope. If you are satisfied, that you have picked up the salivary glands, proceed to dehydrate.
Stain in Borax carmine after 70% alcohol and wash them in 90% alcohol. Rinse them in absolute alcohol, clear in Xylene and mount them in D.P.X. Observe under microscope and draw the diagram as shown below in Fig. 4.10.
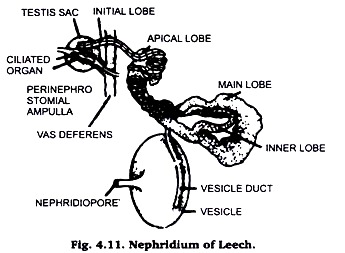
(vii) Nephridium of leech:
After squeezing the leech to remove the blood from its alimentary canal and pinning it down in dissecting tray make a cut throughout the length of the animal along its mid-dorsal line (as described above). Remove the alimentary canal and botryoidal tissue. Locate the dark lateral hazomocoelomic channel and white vas deference running along either lateral slides.
On the outer side of vas deference, in each segment, lies a white coiled nephridium. Removing all attached botryoidal tissue & muscles detch the nephridium from the body wall. Wash it with water in a staining tube and proceed to stain it with Borax carmine. Mount in D.P.X and observe under microscope. Draw the diagram as shown in Fig. 4.11.
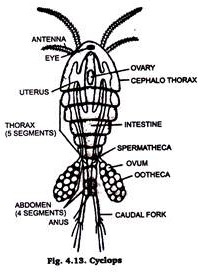
5. Fresh Water Arthropods:
Arthropods are generally represented in fresh water by small sized crustaceans like Raphina Cyclops and many crustacean larval stages. Daphnia (Fig. 4.12) & cyclops (Fig. 4.13) are usually chosen as class work material or preparing permanent slides.
They are killed and fixed in formalin or corrosive sublimate. Formalin is then removed by washing them in water a number of times. Then single staining technique is adopted, with Borax carmine (staining agent) & mounted in Canada Balsam or D.P.X. The prepared slide is examined under microscope. 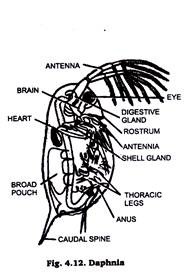
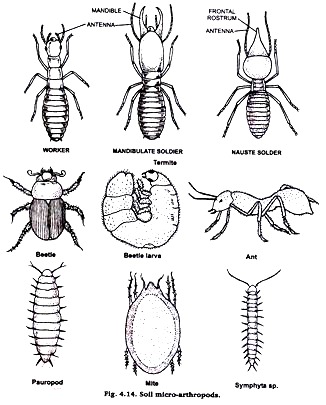
6. Soil Micro Arthropods:
Collection of animals:
Soil micro arthropods are extracted with Tullgren funnel by application of heat. The arthropods are collected, identified and separated. The number of each species is counted for each soil sample and recorded. All the animals are instantly killed & fixed in chloroform. Gently place the specimen in a dish of 5% KOH. When the body becomes somewhat transparent remove KOH. Wash it in water repeatedly to remove KOH.
Take them out one by one, dehydrate with graded alcohol, clear in xylol and mount in DPX for microscopic study. Among soil micro arthropods some are springtial (orchesella sp.), Ternites, Beetles, Beetles larva, Ants, Mite, Pauropods, Syphyla etc. (Fig. 4.14)
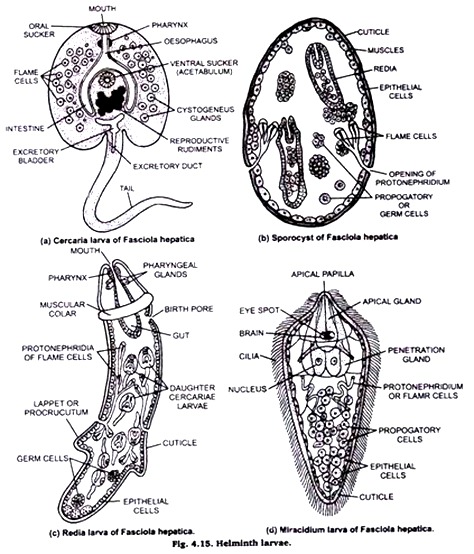
7. Larval Forms of Helminthes:
Collection of larvae:
Miracidium larva of Fasciola can be collected from water sample from the ponds, banks, ditches etc. Where sheep come frequently and snails are also found. After confirmation of the presence of larvae in the water sample they can be collected on greased slide.
Miracidium & cercaria larvae can be collected from the pulmonary sac of the snail also. For Redia & sporocyst the digestive glands should be cut open & then the larvae from the tissues may be collected. Wash the larvae (with host tissue) in 0.78% NaCl saline & separate the larvae from the tissue under microscope.
Isolated larva should be kept then between two slides on the inner surface of which enough grease has been applied Tie the free ends and middle of the slide with a strong thread and put them in acetic formal alcohol.
8. Larval Forms of Arthropods:
Animals of crustacea class include more than one larval stage to complete their life cycle. Nanplins larva zoca larva, metazoea, Megalopa are the free swimming larval stages & given in the class the make permanent slide.
First grease the slides with albumen properly. Put one drop of water containing these larvae. Examine under the microscope to ensure the desired larval stage. Take the larva in the cavity block & kill it with formalin or hot water. Then put the specimen in 5% KOH and larva until internal soft parts dissolve & the body becomes somewhat transparent.
Then carefully draw off the KOH and add water which should be replaced several times to wash out all the KOH. Then dehydrate the specimen through usual series of alcohol using single staining technique and clear in xylol and finally mount on a slide in Canada Balsam or D.P.X. 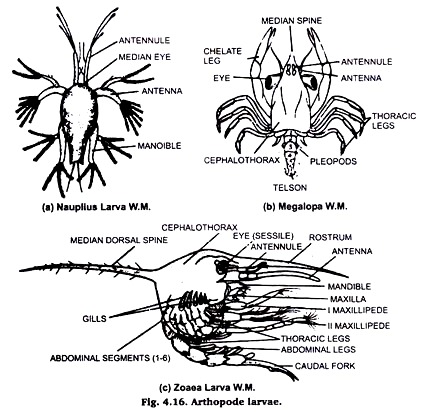
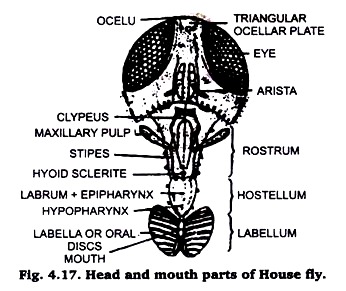
9. Mouth Parts of House-Fly:
It is convenient to use the whole head for study of mouth parts in a fly. Observe the components under a dissecting binocular.
Preparation:
Separate the head of a fly boil it in 5 to10% KOH solution for about 8 to10minutes. Cool it and decant the solution, wash repeatedly with distilled water to remove the last traces of KOH. Mount the whole structure on a slide in glycerin. If required strain in a watch glass and make its permanent slide with single staining technique.
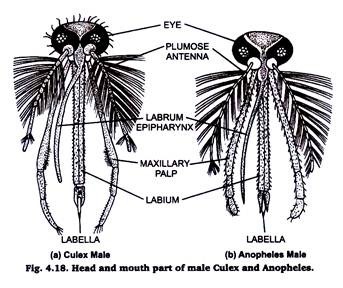
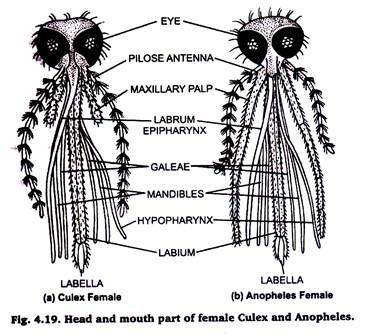
10. Mouth Parts of Mosquitoes:
The basic components of the mouth parts of both the culex and anopheles are same. The size of the proboscis (labium), maxillary palps and antennae differs not only in the two species but also in the male & female members of the same species.
Preparation:
Separate the head of mosquito. Put it in 5 to 10% KOH solution in a watch glass for about 10 minutes. Remove the KOH by repeated washing with distilled water & mount in glycerine.
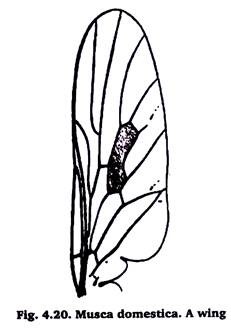
11. Wings of a Fly:
Carefully take out the wings & them in a cavity block containing 70% alcohol. For temporary preparation mount in glycerol. For permanent preparation clear in xylol and then mount in D.P.X. on a slide.
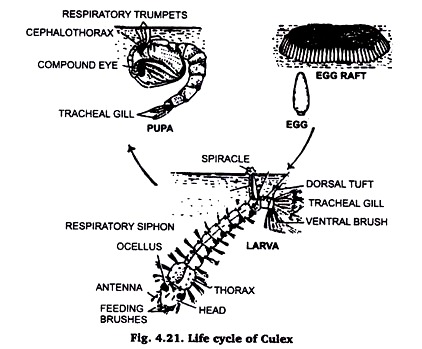
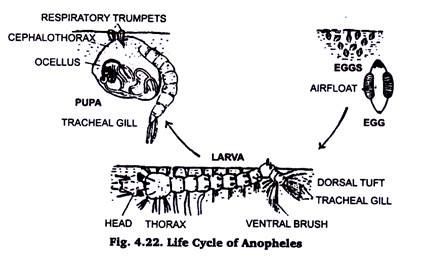
12. Developmental Stages of Mosquito:
Collection of Mosquito larvae:
Mosquitoes spend their larval life in water. The head of the culex larva hangs downward in water, while the Anopheles larva lies parallel to the water surface.
The larvae are collected either with certain amount of water in a container or by fine collecting nets and released in water in a container. If a pot with water is left in a dark corner, mosquitoes will lay eggs there and the larvae will hatch out. The larvae are killed by addition of a few drops of formaldehyde in water.
Preparation of slide:
The larvae are transparent. It is better to stain dehydrate and clear the larvae in a watch glass and mount in Canada Balsam or absolute glycerin. In the later case the cover slip should be sealed with bee’s wax or nail polish.
13. Preparation of Slide of Housefly Pupa (Maggot):
Collect the maggots from dung, manure or excreta & kill them by putting in 70% alcohol or hot water. Then put the specimens in 5% KOH and leave until internal soft parts dissolve and the body becomes somewhat transparent.
Then carefully wash off KOH from water & dehydrate it through usual series of alcohol using single staining technique and clear in xylol & mount on a slide with D.P.X. 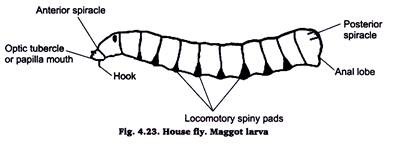
14. Preparation of Permanent Slide of Ticks:
Ticks are small arachnids. These are blood sucking in habit and lead ecoparisitic life on cattle, sheep, goats, horses, men and birds. They are found attached to the skin at the base of hairs or feathers.
To prepare their slide kill them by chloroform & put them in 5% KOH in a watch glass. When the body become somewhat transparent, wash off KOH by water several times. Using single staining technique prepares its permanent slide.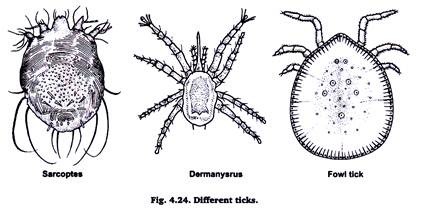
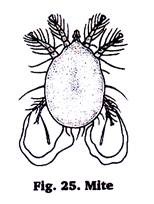
15. Preparation of Permanent Slide of Mites:
Mites are also arachnids but smaller than the ticks. A variety of mites parasitize vertebrates, especially bids and mammals.
Preparation of slides:
Put the pieces of skin containing mites in 10% KOH & scrub to release the mites & leave it in KOH solution for 4 to 8 hours. Centrifuge the contents, discard the supernatant & repeat it several times to wash off skin debries & KOH. Use the single staining techniques, prepare the permanent slide of mites.
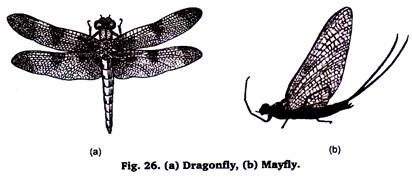
16. Preparation of Permanent Slides of Dragonfly and Mayfly:
Dragonflies and Mayflies are commonly found in the vicinity of lakes, stock tanks and streams. They play an important role in the economy of fresh water. They constitute an important source of food for a number of animals especially fish.
These species can be collected during their swarming flights usually occur between sundown and dark with the help of net trap. To prepare the slide put them in 5% KOH solution for some time. Wash off the KOH and proceed for the procedure of single staining.