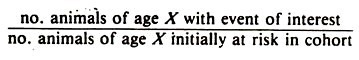Here is a term paper on the ‘Factors Affecting Epidemiology of Disease in Animals’ for class 11 and 12. Find paragraphs, long and short term papers on the ‘Factors Affecting Epidemiology of Disease in Animals’ especially written for college and medical students.
Epidemiology of Animal Disease
Health represents the dynamic balance between the host and its environment. That this balance is frequently tipped against the host resulting in disease is obvious to all; a major role for the epidemiologist is the identification and description of the circumstances and factors leading to the imbalance. Like an ecologist, the epidemiologist is interested in the relationship between the factors (the host and the environment including the agent) and how the relationship changes.
The occurrence of virtually every disease is influenced by factors representing each of the host, environment, and time categories. In addition to humane considerations, the effects of disease on productivity should be a feature when describing the epidemiology of a disease.
During the past century, the study of disease has primarily concentrated on the pathogenesis of disease, and many important epidemiologic features have been ignored. In many instances, the identification of the sources, transmission, survival, and effects of agents of disease was considered as describing the epidemiology of disease.
ADVERTISEMENTS:
However, epidemiologic investigations go beyond the agent and concentrate on the factors of host, environment, and time that alter the occurrence and/or severity of disease for groups of individuals.
In these pursuits, epidemiology is essentially a holistic discipline, whereas most other medical disciplines are reductionists. (This statement is not meant as a criticism of other disciplines, it merely points out two divergent views of health and disease. Society will be served best by cooperation and understanding between the proponents of each viewpoint.)
This article outlines and discusses those factors of the host, environment, and time that should be included when describing the epidemiology of disease; these factors are sometimes referred to as its natural history. Since epidemiology is a pragmatic discipline, it is hoped that subsequent to their identification a means of manipulating causal factors will exist so that the knowledge may be of practical value.
At the very least, a thorough description of the natural history of disease should enhance the understanding of that disease. Often, host factors are of secondary interest in epidemiologic studies. Despite this, it is important to describe the relationship of host factors to disease occurrence and, if necessary, to control the effects of host factors (e.g., by analytic methods such as standardization of rates).
ADVERTISEMENTS:
Otherwise, host factors may distort the observed association between environmental factors and disease. Only when it is known that host factors do not exert a significant effect on the occurrence of disease can they be ignored.
Host Factors Influencing Animal Population:
The major intrinsic host factors are age, sex, and breed. Depending upon the circumstances, other host factors such as species or physiologic state (e.g., pregnancy) should be considered. The occurrence of disease at different levels of these factors is best described by using incidence rates or prevalence proportions, rather than proportional morbidity rates or counts of cases.
As we know that incidence rates and prevalence proportions provide estimates of the risk (probability) of disease occurrence at different levels of the host factor (e.g., in males versus females, intact versus castrated, old versus young, Holsteins versus Jerseys). On the other hand, case counts are influenced by the risk of disease and the number of animals in that host-factor category.
ADVERTISEMENTS:
Thus, the distribution of cases with respect to host factor(s) probably does not reflect the underlying risk of disease. Some veterinary medical texts describe the pattern of disease and make inferences about the risk of disease based solely on the number of occurrences, rather than adjusting for the population at risk.
The reader should be alert to note and hesitant to accept inferences about risk of disease made in this manner. For example, although 60% of all cases of mastitis may occur in 4- to 7-year-old cows, one should not conclude that cows of this age are necessarily at increased risk of mastitis in comparison to other age groups. One must relate the age distribution of cases to that of the source population in order to make inferences about the risk of mastitis.
Sometimes the underlying population rates are unknown and cannot be estimated easily. In these instances the effects of host factors on the occurrence of disease can be described by comparing the relative frequency of the host factor in cases to its frequency in non-cases. A formal statistic for this purpose is the odds ratio.
Age:
Age is probably the most important host variable, because the risk of disease usually is more closely related to age than to other host factors. Thus, age should always be included when describing the distribution of disease. There are many factors, however, that can affect the pattern of disease occurrence with age.
It is important to consider whether the distribution is due to age itself, the current effects of recent environmental exposures on animals of different current ages, or the different past environmental exposures of animals of different current ages. Whether age per se actually changes the risk of disease independent of the environmental factors is unknown.
However, epidemiologists attempt to identify environmental factors that accompany but are separable from age that may alter the risk of disease. For example, the cumulative insults of machine milking may provide a more reasonable explanation for the progressive increase in risk of mammary gland infection as cows become older than age per se.
Such a hypothesis is quite easily tested and if support for it is found, better milking machine design and/or more careful milking techniques should provide methods of preventing at least some of the increased risk related to age. Some unavoidable and unalterable changes in the mammary gland due to aging may persist however.
If age-specific rates are plotted (e.g., as a histogram), the shape of the resultant plot will depend on whether morbidity rates (incidence or prevalence), mortality rates, or the rates of other intermediate events such as culling are used. If age exerts a major influence on the risk of the disease in question, one would expect either a uniform increase or decrease —not necessarily linear —or a unimodal pattern of disease occurrence with age.
ADVERTISEMENTS:
For example, data on the occurrence of a number of syndromes in dairy cattle indicate that the incidence rate (risk) of most diseases increases with age. For many of the diseases the increase is consistent with a linear trend, whereas for others the pattern is curvilinear. Some diseases (e.g., various pneumonias in cattle) have a U-shaped age pattern.
That is, the disease occurs relatively frequently in young and old animals — probably either because of recrudescence or increased susceptibility —but with low frequency during the middle years. This pattern would probably be more pronounced if cohorts of cattle were followed from birth rather than from first calving.
Often, only prevalence data from periodic surveys are available, and this makes it difficult to determine the risk of acquiring infection or disease by age. Formulas are available to estimate age incidence from prevalence, but most are based on the assumption that the substance being measured (usually antibodies) is present for the life of the animal and that immigration and emigration in the population are minimal.
Based on these assumptions, a constant probability (risk) of acquiring infection with age will produce a curvilinear age-specific prevalence pattern that increases with age. Similarly, it is difficult to make inferences about the risk of infection based on age-specific disease rates, or to make inferences about the risk of disease based on age-specific mortality rates. Nonetheless, knowing the age pattern of disease and mortality can help generate useful hypotheses about factors that might influence infection and disease respectively.
If the pattern of disease occurrence with age appears to be bimodal (i.e., two peaks are present), this may indicate that there are in fact two distinct syndromes present— although they may have clinical or pathological similarities —or that factors influencing disease occurrence in the different age groups differ. Apparently, bimodal patterns exist for feline leukemia and canine progressive retinal atrophy.
Infectious bovine rhinotracheitis (IBR) virus is associated with a number of syndromes, the frequency of which produces a bimodal or trimodal pattern with age. In calves less than 1 month of age, the virus produces an enteric syndrome. In older calves, 6-18 months of age, an upper respiratory tract syndrome is seen.
In adult females, the virus is associated with both infertility and abortions. Since the same virus is common to these different conditions, the different syndromes probably reflect changes in the physiologic condition of animals as they age and differences in environmental conditions, rather than differences in the virus itself.
In humans, the young have a higher risk of most infectious diseases than do teenagers or adults, because the latter have an acquired immunity due to past exposure to the agents of these diseases, and because of physiologic and behavioral changes with age. Despite the higher rate of occurrence in the young, the severity of disease (chiefly under host control) often is less in the young than in the old.
This is particularly true if the initial exposure of the young occurs while they have passive protection. The level and duration of passive protection in the young depend chiefly on the extent and timing of exposure of their mothers to the agent.
When infections such as measles or poliomyelitis enter populations that have not been exposed for a number of generations, the differential rate of occurrence with age is absent and the increased severity of the disease in mature people becomes apparent.
The above age-related phenomenon probably occurs in animals also, but the pattern may be obscured for a number of reasons.
First, a large percentage of domestic animals are slaughtered prior to reaching an age equivalent to adulthood in humans.
Second, the hygienic standards on most farms facilitate the early exposure of animals to the more common pathogens, and vaccination programs may have altered the pattern of resistance in both adult and young populations.
For example, if one observed cohorts of feral cats, the pattern of diseases such as pan-leukopenia would probably be quite different than the pattern in domesticated felines.
These differences would reflect the divergent environmental exposures of these groups of cats, as well as possible inherent host differences such as genotype. Severe outbreaks of disease (such as infectious bovine rhinotracheitis or bovine virus diarrhea) in closed herds probably represent an analogy to the “island” outbreaks of human measles.
Maintaining closed herds may free the owner from the everyday problems of endemic diseases; however, additional vigilance is required to prevent serious outbreaks following the introduction of infection to this highly susceptible population.
Most measures of productivity also are age related; examples range from racing ability in horses to milk production in dairy cows. Young animals appear to be more efficient than adults at converting feed energy into usable products, be it eggs or muscle protein. Despite this, there may be economic value in prolonging the life of certain animals.
For example, the average survival time of dairy cows in Canada is about 4 years after their first calving. Since a cow’s production potential does not decrease markedly between 7 and 10 years of age, the dairy industry might benefit if diseases leading to premature involuntary removal from the herd could be prevented.
This is particularly true because of the large investment in rearing replacements for swine, beef, and dairy herds. Of course, this potential benefit must be balanced against the increased opportunity for genetic improvement afforded by replacement of culled stock.
The economics of culling and purchasing or raising replacements should also be considered. Because of the marked and consistent effect of age on the absolute level of production, some parameters (e.g., milk production in dairy cows) are standardized to facilitate direct comparisons of production in animals of different ages.
Sex:
A number of diseases are associated with the sex of animals; for example, infectious diseases may occur more frequently, or with greater severity, in young male humans than in young females. On the other hand, female dogs have a much greater risk of diabetes mellitus than males. This is also true of humans, and is an indication that dogs may be a good model for studying the pathogenesis of diabetes.
Many of the sex-associated diseases are directly or indirectly related to anatomic and/or physiologic differences between the sexes. Such diseases include parturient paresis (milk fever), mastitis, metritis, and cancer of the mammary glands in females, as well as sex-related behavioral problems such as abscessation as a result of fighting and urine spraying in male cats.
Neutering also may be associated with disease occurrence. These associations range from a sparing effect on the risk of mammary gland cancer in spayed bitches to an increased risk of laminitis in castrated ponies, the latter also being related to behavioral and husbandry changes.
The risk of the feline urologic syndrome is increased in castrated males; however, not all of this increase is likely due to anatomic changes because spayed females also have a higher risk than intact females.
When investigating the effects of neutering, the age at neutering should be considered. Sex of the animal also needs to be taken into account when productivity is being evaluated, since racing ability, weight gains, deposition of body fat, and feed efficiency may differ between sexes.
Breed:
Breed differences in risk of disease and level of productivity are common, and breed effects should be considered and controlled (adjusted for) when studying the effects of other factors on disease occurrence or productivity. In general, breed differences may be separated into two components: differences due to genetic factors and differences due to phenotypic factors.
Population genetics, like epidemiology, is highly dependent on the collection and analysis of data from observational studies. Both disciplines are interested in determinants of disease, and as it is often unclear at the outset whether a disease has genetic determinants, there is much overlap between the two disciplines.
Animal geneticists have developed a set of specialized analytic methods for identifying the heritability of continuous production traits. Recently these techniques have been modified to study the heritability of discrete traits such as the presence or absence of disease.
The equivalence between these techniques and epidemiologic statistics such as the population attributable fraction remain to be clarified. Although still in its infancy, one thing is clear: few diseases are determined solely by genotype or environmental factors. In fact, our current genetic make-up is a result of the selection pressures exerted by the environment on our ancestors.
The close relationship between genetic and environmental determinants may be demonstrated by two avian diseases, yellow shanks and pendulous crops. Yellow shanks occurred when poultry with a specific genetic defect were fed yellow corn. If a farmer had only genetically defective birds and fed both yellow and white corn, the ration would appear to be the determinant, since only those fed yellow corn would develop yellow shanks.
If a farmer had normal and genetically defective birds and fed only yellow corn, genotype would appear to be the determinant, since only genetically defective birds would develop the condition. In this syndrome both factors are required to produce the disease, and in the syntax of sufficient causes, the genetic defect and the specific environmental factor (yellow corn) would be considered necessary components of the sufficient cause (i.e., both must be present for the disease to occur).
Pendulous crop in turkeys is slightly more complex in that three factors —genotype (bronze turkeys), environment (very hot weather), and excess water intake —combine to produce the syndrome. Assuming no restriction on water intake and only bronze turkeys being present, the disease appears to be environmentally determined. If two or more breeds are raised under hot conditions, the disease appears to be genetically determined.
Again, genotype and environmental factors are components of a sufficient cause. Phenylketonuria represents an analogous disease in children in that both environmental and genetic factors are involved. In these examples, because both factors (genotype and environmental) are required for the disease to occur, the interaction between genotype and environmental factors is said to be complete.
The relationship between genotype and environment as determinants of many diseases is often not as obvious as in the previous examples. If the disease has determinants other than a particular genotype-environmental factor combination, the statistical interaction between genotype and environment is less than complete.
Although feasible, it is more difficult to identify putative causes in these instances. In general, the sensible approach would be identifying the role that each factor plays as a determinant of the disease, and using this knowledge to prevent the disease in so far as the factors can be manipulated.
In some cases, diseases initially considered to be genetic in origin were later shown to be essentially determined by environmental factors. For example, detailed experiments were conducted to prove that a particular cyclopean malformation in sheep was caused by a genetic defect. The experiments failed, and later observational and experimental studies identified a poisonous plant, Veratrum californicum, as the major cause.
In retrospect, careful analysis of the available observational data might have convinced the investigators of this without the need of expensive experimental studies. In this case, as well as in early Texas fever investigations, the observations of ranchers were eventually validated, although veterinary investigators initially ignored and sometimes ridiculed the initial observations.
Diseases due to genetic defects such as baldy calves, dwarfism, and spastic paresis in bulls (most following a Mendelian inheritance pattern) have been identified.
The heritability of diseases following more complex patterns (e.g., Galtonian characteristics) has not been studied as well as the simpler Mendelian type characteristics. Certainly, resistance to infectious disease has a genetic component as demonstrated by experiments with laboratory animals (e.g., the selection and breeding of leukosis-resistant strains of poultry and Aleutian disease resistance in mink).
However, identification of the heritability of most diseases of domestic animals must await the development of large, accurate data bases, similar to those currently available for recording production. Preliminary work suggests that diseases such as mastitis, atrophic rhinitis, and cystic ovarian disease have a genetic component, and that their heritability is sufficiently large so that sire and dam selection could reduce their incidence rate.
Also, data from some swine herds indicate that the variability in the mortality rate in litters due to sire is quite large, varying from two to six times. This suggests that sire selection could reduce piglet mortality significantly.
In companion animals, the risk of many diseases including cancers, arthritis, and heart defects varies greatly among breeds. However, the proportion of this difference in risk that is genetically based is unknown. For example, phenotypic factors probably alter the risk of diseases such as hip dysplasia, with large breeds having an excess risk.
Yet, there is a significant variation in the risk of hip dysplasia among dogs of the same general phenotype, and more than 25% of certain low-risk phenotype breeds develop dysplasia. Both of these facts suggest an important role of genotype. It has been shown that for some breeds, dogs owned by one person have significantly higher or lower rates of hip dysplasia than the breed average.
This again supports the potential role of genotype and/or shared environment as determinants of this disease. To further complicate the issue, the effects of genotype and phenotype on the risk of hip dysplasia appear to be partially confounded with environmental factors, such as the amount of exercise the dog receives when young.
Phenotypic factors are believed to be important determinants in a number of diseases, ranging from bone cancer in dogs to displaced abomasum in dairy cows. A lack of pigmentation increases the risk of cancer-eye in Hereford cattle whereas gray coloration increases the risk of melanoma in horses. The underlying reasons for these associations are unknown in most cases.
Data bases will be available in the near future that should allow an assessment of these types of multifactor problems. For example, one should be able to assess the impact of sire, phenotypic factors (e.g., size of cow, depth of chest, shape of abdomen), and other variables such as calving ease (which may be related to size of pelvic inlet and size of fetus) on the risk of abomasal displacement and other diseases.
Dogs have been and will continue to be studied intensively to aid understanding of the role of genotypic and phenotypic factors on disease occurrence and to identify models of human diseases.
No other domesticated species has such a wide range of genotype and phenotype, and dogs share man’s environment intimately and the occurrence of their diseases (such as bladder cancer) may be indicative of toxic substances in the environment of potential danger to man.
The host factors can distort the association between disease and factors of more immediate interest. For example, female canine diabetics were 16 times more likely to have a diagnosis of benign mammary tumor than female dogs with other endocrine diseases.
When a summary statistic (odds ratio) adjusted for age was calculated (using the Mantel-Haenszel technique) the odds ratio was reduced to 5.6. This technique is used frequently in analytic studies to control for the effects of extraneous factors.
Environmental Factors Influencing Animal Population:
The environment includes all the biotic and abiotic components of a place, be it a pen within a barn or a large geographic area. Knowledge of the rate or risk of disease according to place is a first and essential step in understanding disease. In initial investigations the number of potential differences between areas where disease is frequent and where it is infrequent is so large that only general theories can be developed to explain its distribution.
Subsequently, more detailed investigations of specific components of the environment may be pursued. General categories of environmental factors include features of the landscape or place, abiotic elements (i.e., air, soil, water, and climate), and biotic features including the flora and fauna.
Immediate causes of disease, whether living organisms or toxicants, should also be sought and their importance as causes of the disease quantified. Whether one should concentrate initially on general features, such as air quality or the plant life of an area, or on the identification of specific agents depends on the setting and the nature of the problem.
As a general suggestion one should not concentrate interest on specific agents to the exclusion of studying more general features of disease occurrence. Often, knowledge of the general features provides useful guidelines in generating a logical series of hypotheses about the involvement and nature of specific agents.
As an example of this approach, consider multiple sclerosis in humans, the ultimate cause of which still eludes researchers. The frequency of multiple sclerosis is directly correlated with latitude and increases dramatically with distance from the equator.
Thus, one major thrust to current epidemiologic studies is to concentrate on cohorts of people who either enter or leave high- or low-risk areas. Since the change of risk of disease in these individuals appears to be related to their age at migration, the presence of a specific agent in high-risk areas is suggested.
When disease occurs more frequently in certain areas than in others, the disease is said to be clustered. Disease may be limited geographically for a variety of reasons, many of which relate to forces that act upon the host, vector, or agent of disease.
Geographic features such as rivers, lakes, and mountains can also serve to restrict the spread of disease. Sometimes disease is limited to traditional migration or market routes; this was true of Texas fever (bovine piroplasmosis).
Although usually large, the geographic area of interest can vary in size from pens within a barn, to barns within a farm, to areas within a country. A recent serial provides data on the distribution of a variety of diseases and disease outbreaks in countries throughout the world.
Historically, knowledge that certain geographic markers (e.g., bogs or marshes) were predictive of increased risk of disease was used to prevent disease simply by avoidance of these areas. Some diseases like swamp fever (equine infectious anemia) are named after their association with these geographic features.
During the 1800s, the observation that the distribution of Texas fever was analogous to that of the tick suspected of spreading the disease was instrumental in gaining support for further study of the role of the tick.
The tick was subsequently shown to be the reservoir of the agent and capable of passing the infection by vertical transmission to succeeding generations of ticks. More recently, the study of the association between agents of disease and certain ecosystems or geographic markers has expanded and has been termed landscape epidemiology.
There are a number of ways of determining spatial clustering, many of which are based on fairly rigorous mathematical procedures. For most practical purposes, simple graphic methods of detecting clustering are suitable. These include cartographic techniques such as spot maps, transparent overlays, isodemic maps and grid maps.
Cartographic Methods:
Spot maps are a basic tool for studying the geographic pattern of disease. Each occurrence of disease is plotted on a standard map, the scale of which depends on the investigation.
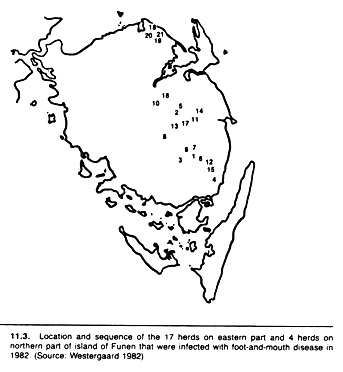
Spot maps can be modified to show the change in distribution of disease over time. For example, different colors can be used to plot the occurrence of disease during different time periods, or each spot may be numbered to indicate the relative time of disease occurrence (see Fig 11.3).
Sometimes, instead of plotting each case individually, the average level of disease on a farm or in an area may be represented by different types of markings on black and white maps, or different colors on colored maps. Adjusted rates or, more frequently, standardized morbidity or mortality ratios may be plotted rather than unadjusted rates (host factors that may affect the level of disease are usually included in this adjustment).
Although too elaborate for routine use, three-dimensional, computer-drawn maps (with the height proportional to the level of disease) provide tremendous insight into the geographic distribution of disease. The fox population of Switzerland has been displayed using this technique.
Transparent overlays are also useful in mapping disease. One could describe the spread of a disease (such as rabies across a country) by plotting the extent of rabies in different time periods on separate transparencies; then the spread of disease can be displayed by sequentially overlaying the transparencies.
Grid mapping is not particularly useful for the practitioner, but it is useful when maps may be drawn using data in computer files. In this instance, each particular location is referenced by a specific x – y co-ordinate (a longitude and latitude marker).
Using this technique, large volumes of data about the location of specific cases can be stored easily in computer files, and the same files can be utilized to create the map. The files may be updated regularly and maps easily redrawn as required.
One’s ingenuity is the only real limitation to the usefulness of cartographic techniques. However, since the population at risk is frequently not uniformly or randomly distributed, one must be careful in interpreting clustering if only the distribution of cases is plotted. Steck and Wandeler (1980) provide many good examples of the use of cartographic techniques.
Isodemic mapping is a cartographic technique used to correct for non- random distribution of the population at risk. In ordinary maps, the area of different portions of the map reflects the actual physical area of the administrative unit. Thus, two counties of equal geographic size will be represented by equal-size areas on a map.
In isodemic mapping each administrative area (e.g., a county) retains its original shape, but the size of each area when mapped corresponds to the relative magnitude of the population at risk, not the actual physical size of the area.
Analytic Methods:
Often, a plot of infected premises may indicate clustering, suggesting farm-to-farm spread. However, in the absence of data on the distribution of all farms in the area such clusters are difficult to interpret.
One way of assessing this apparent clustering is to compare the average distance between any two infected farms to the average distance between two randomly-selected non-infected farms, or to the distance between randomly- selected non-infected farms and the closest infected farm.
The distance may be “by road” or “as the crow flies” depending on the situation. If automobiles or trucks are suspected of spreading the infection, road distance would be used. (This would not preclude the tracing of known vehicle movement and relating this to the distribution of affected farms.)
If airborne spread were suspected, a straight-line distance would be more suitable. The latter can be obtained using calipers to measure the distance on an accurately plotted map of appropriate scale. If farm-to-farm spread is an important means of transmission, one would expect the average distance between pairs of infected farms to be less than the average distance between a non-infected farm and the closest infected premises.
A similar method was used in a case-control study of brucellosis in two counties in Ontario, Canada. The case farms were infected farms identified from the district regulatory veterinarian’s records. The controls were obtained by taking a random sample of herds with negative tests, provided the tests were conducted during the time period selected for the study.
The average distance between the two closest infected farms was less than the distance between a non-infected and the nearest infected farm, supporting the hypothesis of farm-to-farm spread. This technique will not discriminate between “fence-line” and airborne spread, but it does provide an indication of whether the clustering is an artifact due to the distribution of farms or a real phenomenon.
Interpretation of Clustering:
Once a relationship between a disease and geographical areas has been documented, it should be studied to see if characteristics of animals in the area can explain the association.
If an explanation in terms of host factors cannot be found, the following observations provide additional evidence that factors localized to a geographic area may be responsible for the association: animals leaving the high-risk area subsequently develop a lower risk of disease, and healthy animals entering the area experience an increased risk of disease; most animals of the herd or species of concern have a high rate of disease in the suspect area, and animals of the same breed or species do not have high rates of disease outside the suspect area; and animals of different breeds or species all have an increased rate of disease in the high-risk area.
The latter observation is supportive but not essential, because only one breed of animal may be at risk of the disease. This could be due to inherent behavioral traits of the breed or to the system of husbandry imposed on it.
Temporal Factors Influencing Animal Population:
Just as the occurrence of disease is related to host and environmental factors, there are changes in the frequency of many diseases with time. These temporal patterns of disease occurrence should be elucidated clearly and detailed explanations for them sought using formal studies.
In this section various graphic methods used to identify the pattern of the temporal changes in disease frequency are presented. Knowledge of temporal patterns may provide insight into factors affecting the balance between the host and agent. For example, in outbreaks of disease, the pattern of change (particularly its abruptness) may suggest an optimal method of investigation of the outbreak.
General Temporal Patterns:
When plotting the number of cases or the rate of disease against time, the shortest practical time scale should be used. If the disease occurs infrequently and without discernable pattern, it is classified as sporadic. If the disease occurrence has a predictable pattern, it is classified as endemic.
Seasonal or cyclical fluctuations in disease occurrence do not preclude the correct use of the term endemic, so long as the changes are predictable (i.e., occurring with regularity). The average frequency of endemic diseases may be low (hypo-endemic), moderate (mesoendemic), or high (hyper-endemic).
If the level of disease occurrence is significantly greater than usual (more than two standard deviations above average) and the increase is not predictable, the disease pattern is classified as epidemic. If the epidemic occurs throughout a number of countries, it may be termed pandemic.
The three patterns of disease occurrence (sporadic, endemic, and epidemic) provide useful information about the host-agent balance. Sporadic patterns suggest that the agent either infrequently infects the host, or the agent is usually present and clinical disease results from the effects of other factors.
Clinical mastitis in dairy cows and infectious thromboembolic meningoencephalitis in feedlot cattle are diseases which occur sporadically. The infectious agents of these diseases usually are present, but clinical disease occurs infrequently and is not readily predictable.
Some evidence indicates that meningoencephalitis tends to be associated with outbreaks of respiratory disease, and the stress and physiologic changes resulting from the respiratory disease may allow the Haemophilus organisms to enter the circulatory system, subsequently producing lesions in the central nervous system.
It could be argued that a large percentage of infectious diseases seen by veterinarians are sporadic in nature and probably result from unknown factors tipping the agent-host balance in favor of the agent, rather than from intrinsic properties of the agent per se.
Endemic diseases are a result of a predictable, probably long-term balance between the agent and host. The lower the level of disease (degree of endemicity), the better the balance between the host and agent. The balance is quite dynamic, however, and both the level and the stability of the balance can be influenced by environmental as well as host factors.
Subclinical mastitis is mesoendemic in North American dairy cows and dairy calf mortality is mesoendemic in California dairy farms. The increase in disease (chiefly respiratory disease) that occurs after feedlot cattle are assembled should also be termed endemic because of its predictability.
The level of endemicity is less certain, but it appears that management is a major determinant of it. Although it is almost always fatal for individual foxes, rabies is endemic in the Canadian fox population and increases in occurrence, quite predictably, in the fall of each year when the fox kits leave their home and search for new territory.
Epidemic patterns suggest a gross imbalance with the agent having the upper hand. This imbalance is common when a new strain of organism is produced (e.g., by mutation), or during the initial exposure of the host to an organism. Currently, no adequate explanation of the pandemic of canine parvovirus enteritis exists.
All the above patterns of disease with time relate to explicit geographic limits. Diseases (such as foot-and-mouth disease) that may be endemic in some areas of the world, may produce epidemics in other areas, even though the number of cases in the epidemic area might be far less than in the area designated as endemic.
It is a general epidemiologic tenet that over time the relationship between a host and agent changes from the parasitic (favoring the agent) to a commensal state (favoring neither host nor agent). Thus, given time and a stable environment, the pattern of disease changes from epidemic to endemic and finally to sporadic. In the natural state the more resistant hosts have an increased probability of survival.
From an ecologic viewpoint, the production of disease or death rarely favors the perpetuation of the agent; thus natural selection favors less pathogenic organisms. Rabies and plague are notable exceptions to this rule. Thus, although in the short-term there usually is a positive correlation between the level of infection, disease, and death, this will not likely be true over a long period.
Rather, the number of cases or deaths relative to the number of infected animals declines with the passage of time. Under laboratory conditions it is possible to select for increased virulence by repeated passages of the agent, usually in the same species.
This does not contradict the previous principle, and is primarily due to the unnatural selection—if the previous process is called natural selection —of the sickest individuals for culture and repeated passage of the isolated agent. Under these restricted artificial conditions, the more virulent strains of organisms have a marked selection and survival advantage.
The history of the biological control efforts aimed at the European rabbit, Oryctolagus cuniculi, in Australia provides an excellent opportunity to examine the evolution of a host-parasite relationship. The rabbit was introduced into the southern part of Australia by Thomas Austin in 1859.
In the ensuing years, because of the lack of natural predators, it advanced at a rate of approximately 70 miles per year over large parts of the country. By 1887 the rabbit population had multiplied so proficiently that the government offered a reward for a method that would exterminate it.
Although it had been previously observed that myxomatosis was very lethal for Oryctolagus, the first to suggest the use of myxoma virus as a method of biological control was a Brazilian investigator named Aragao. Experiments were subsequently carried out to determine whether the virus would be harmful to other Australian animals.
It was not, and myxoma virus was deliberately introduced into the rabbit population in 1950. Within 10 months, infected rabbits were found over an area of approximately 500,000 square miles. By the third year following virus release it was estimated that the original rabbit population of approximately 500 million had been reduced by 80-90%.
However, within several years of its initial release, the virus being isolated in the field was less virulent (the case fatality rate decreased from 99% to approximately 90%), and the time between infection and death had increased.
Change in the resistance of the rabbit was slower to develop but was also evidenced by 1957. By this time, the rabbit population in some locations had been exposed to five successive epidemics each having at least a 90% case fatality rate.
Using virus that killed approximately 90% of rabbits selected from previously unexposed areas, the case fatality rate in the latter repeatedly exposed population was less than 50%. This degree of protection was not due to any acquired immunity due to previous exposure, as the vast majority of these rabbits and their parents had never encountered the virus although their ancestors had.
The changed resistance was innate and inheritable-an example of natural selection in a very intensive form acting to favor gene mutations. By 1965 it was estimated that the rabbit population and the virus had evolved to a state with the rabbit population at around 20% of their numbers before the advent of myxomatosis.
Graphic Techniques:
The temporal patterns of morbidity and/or mortality may be investigated and displayed by appropriate graphic techniques. Initially, one can plot the number of events of interest or, more preferably, the rate (incidence, prevalence, or mortality) against time. Patterns may be obvious at that point. By general agreement, secular trends describe changes over many years or decades; cyclical changes are those with a periodicity of 2-5 years; and seasonal changes have a periodicity of 1 year or less.
Often the random variation in disease occurrence can obscure temporal patterns. A technique known as a moving average is useful to remove the unwanted fluctuations and allow visual identification of any underlying patterns. Moving averages of 3 to 5 months are useful for investigating seasonal patterns; 15- to 25-month moving averages for cyclical patterns; and 37-month moving averages for long-term (secular) trends.
To plot a 3 month moving average, the rates for January, February, and March are averaged and plotted against February, the temporal midpoint for the average. Then, the rates for February, March, and April are averaged and plotted on March. This continues until all the data have been included. Obviously, many years of data are required to adequately identify cyclical or secular patterns.
When interpreting secular changes, one should look for marked trends or abrupt changes, since useful explanations (hypotheses) often may be found. When attempting to explain the changes, it is important to assess whether other factors (e.g., differences in diagnostic accuracy, completeness of reporting, changes in duration of disease, differences in host characteristics) can explain the disease pattern.
Indications that the trend is real may be found by identifying different trends in different breeds, or different trends in different diseases of equal diagnostic difficulty.
In addition, if a disease is not fatal, its prevalence among necropsied animals and/or abattoir specimens over a period of time can be used to assess long-term changes. Gradual changes in disease occurrence are difficult to interpret and rarely suggest useful explanations for the change because a large number of differences (particularly in environment) may have occurred during that time.
When cyclical changes are noted, a likely explanation is that herd immunity underlies the pattern. This might involve alterations in the immune percentage (due to lack of exposure or the birth of susceptible animals) or changes in the probability of contact, possibly because of variations in population size.
Seasonal variations in disease occurrence may have a number of different causes, ranging from direct effects of weather on the agent or host, to indirect effects of weather due to changes in flora and fauna, or to management and housing changes of animals in relation to weather.
Diseases in which wildlife with seasonal habits serves as reservoirs or carriers and those transmitted by insect vectors tend to have seasonal patterns. It is also possible for dramatic yearly increases in the susceptible population to lead to seasonal patterns of disease.
This may explain the seasonal occurrence of feline pan-leukopenia. However, usually more than one birth cohort is required to increase the number susceptible to the point where a disease outbreak is likely to occur. This would explain the 2-5 year periodicity for cyclical changes.
Age and Time Interrelationships:
The patterns of disease with age can assist in generating hypotheses to explain disease occurrence. However, care is required when interpreting these patterns. The existence or occurrence of an event (i.e., disease, death, or culling) may be affected by age per se, and/or by factors acting temporally close to the occurrence of the event (the current environment), and/or by factors that existed at some time prior to the occurrence of the event (the past environment).
For example, the current milk production of dairy cows is related to the probability of being culled and may be influenced by current age, current environmental factors (such as the presence or absence of mastitis in the herd) and past environmental factors (such as whether or not the cows had pneumonia as calves). The problem is to identify which of these factors plays an important role in the level of production and hence of culling.
The usual method of examining age patterns (such as those of culling) implicitly relates the occurrence of the event to a current time period; that is, the rates portray the age pattern of occurrence currently existing. This method of calculating rates has been called periodic, cross-sectional, or current; the latter being preferred here.
Current rates for a specified calendar time period have the following general form which is similar to that used for most rates:
The formula may be modified depending on what is being studied (i.e., prevalence or incidence, mortality, culling). When interpreting current rates, assume the event of interest is influenced by the current environment; however, the effects of age cannot be separated from the effects of the current environment, and the effects of past environment must be ignored.
If the age pattern of disease occurrence could be influenced by past environmental experience (including the animal’s history with regard to previous disease occurrence) another approach known as cohort analysis is useful. Cohort analysis describes the rate of the event of interest in a defined cohort over a series of time intervals.
Cohort analysis uses rates calculated as for risk rates and have the following general format for each time period:
Again, this formula should be modified depending on what is being measured. All the animals in the numerator are a subset of the initial cohort of animals. Cohorts are usually defined on the basis of time of birth (month or year), time of entry to the herd, or on the basis of experiencing an event of interest such as parturition.
To separate age effects from effects of current environmental factors and from effects of past environmental factors, the results from at least three surveys conducted in different calendar time periods should be available.
Age effects are present when the disease pattern varies by age, regardless of cohort; cohort effects are present when the disease pattern varies by cohort, regardless of age. Current effects are present when the disease pattern varies by calendar time regardless of age and cohort.
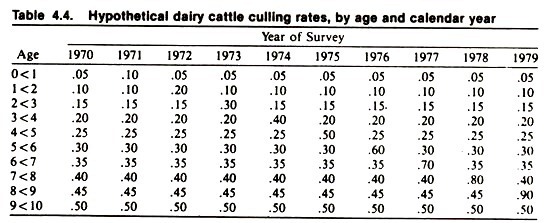
An example of this approach (using fictional data describing current culling rates in dairy cows, based on a series of yearly surveys) is given in Table 4.4. Consider the data relating to 1973; note the general increase in the rate of culling with age, and the peak in the 2- to 3-year-old cows.
An interpretation of the increased risk of culling with age might be that cows “wear out” as they get older. The peak in the 2- to 3-year-old cows might be explained as an age effect (cows are more likely to be culled in their first lactation) or that environmental factors existing in 1973 exerted a greater harmful effect on 2- to 3-year-old cows than cows of other ages. It is not possible without additional data to discriminate between these possibilities.
Suppose that in 1978, another periodic study of culling was performed in the same population. Again, note the general increase in rate of culling with age, and the peak risk in 7- to 8-year-old cows. How does one interpret this peak? Is it an age effect or is it due to current environmental factors being particularly detrimental to the survivorship of 7- to 8-year-old cows? The answer is not obvious.
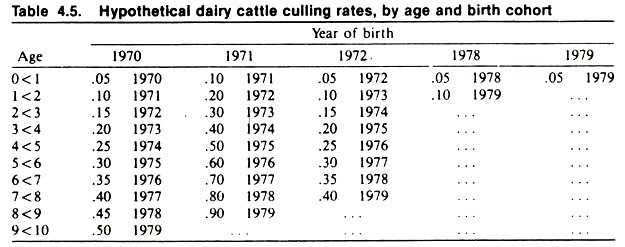
Since the past environment of cows might affect the current probability of culling, it is desirable to examine rates based on the cohort approach. As in this example, the cohorts usually are defined and the cohort rates calculated, retrospectively, from the available data.
The culling rates of each birth cohort are shown in Table 4.5. Note the general increase in risk of culling with age, similar to what was observed in the current surveys. Note also that the cohort born in 1971 has twice the risk of culling of other cohorts.
Now, armed with the results of both approaches, it is easier to logically interpret the effects of age and current and past environment on culling. Since the risk of culling increases with age in the cohort approach, it seems logical to accept this as an underlying biologic association.
Also, since the increased risk of culling in the 1971 birth cohort explains the peaks noted in the 1973 and 1978 surveys, it seems reasonable that factors active in this birth cohort of calves (perhaps an outbreak of enteric or respiratory disease with permanent tissue damage) explain the peaks of culling. (The disease pattern is consistent in this cohort regardless of age.) Since no other patterns are noted in the cohort rates, one may conclude that the current environment had little effect on culling.
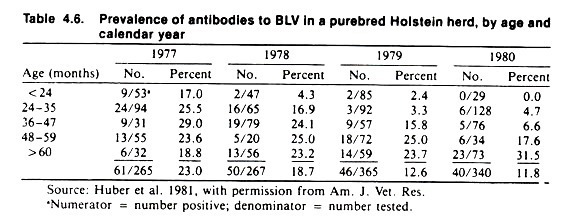
Usually, the patterns of disease are not as clear as those given in this fictional example; however, veterinarians should realize the potential value of the cohort approach. Table 4.6 contains the results of four current surveys, conducted at yearly intervals, to determine the reactor rate to bovine leukemia virus (BLV) in a dairy herd.
Notice that the prevalence proportion decreases with time from 23% in 1977 to 11.8% in 1980. (This feature is sufficient to indicate that both current and cohort analyses should be used. If there is no secular trend in the frequency of the event of interest with time, the age pattern will be the same in both the current and cohort approaches, as it was in the previous example of culling.)
The reactor rates also appear to increase with age, except for the lack of an obvious pattern in 1977. Assuming these change reflected the effect of current environment and age, the changes are consistent with horizontal spread of an endemic infection.
That is, the older animals get, the more likely they are to have contacted the endemic infectious agent and have antibodies to BLV. An explanation for the decrease in prevalence with time is not obvious.
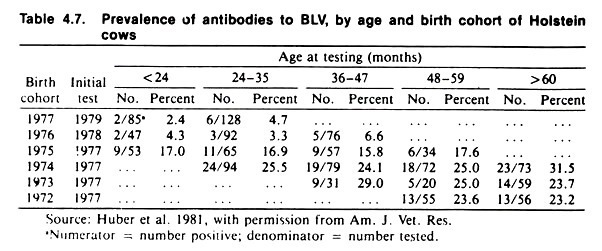
Table 4.7 portrays the same data using a cohort format. Note that there is only a slight increase in prevalence, according to age, within each cohort. (The cohorts are birth cohorts, but due to the method of testing there are missing data; some cohorts were 4-years-old before they were tested.) Note also that the prevalence proportion decreases in the more recent cohorts.
Taken together, the results of the current and the cohort analyses imply a large cohort effect, a small increase in prevalence with age, and no effect of current environment. (Recall the conditions described earlier for age, cohort, and current effects.) There appears to be minimal spread of infection among cohorts in this herd.
Why each succeeding cohort should have a lower prevalence of reactors than its predecessor (in the absence of a control program) is an interesting question to ponder; although there is no obvious explanation for it, the cohort effect is nonetheless real.
These data are not intended as the final word on BLV in dairy herds. Many people believe (primarily based on current rates) that the prevalence rate increases with age as a result of horizontal transmission of the virus. A recent prospective cohort analytic study investigated the time(s) at which horizontal spread of BLV appeared greatest; the data from this study indicated an increasing prevalence of BLV antibodies with age (i.e., a true age effect).